Structural Research of Glycoproteins
Glycoproteins, a class of proteins that bear covalently attached carbohydrates, play crucial roles in various biological processes, including cell recognition, signaling, and immune response. Understanding the structural characteristics of glycoproteins is essential for unraveling their functional mechanisms and developing targeted therapeutics. In recent years, significant progress has been made in the field of structural research on glycoproteins, shedding light on their intricate molecular architectures and functional implications.
Glycoprotein Structure: Unraveling the Complexity
Glycoproteins are characterized by the presence of glycans, which are branched carbohydrate chains attached to specific amino acid residues within the protein backbone. The structural analysis of glycoproteins poses unique challenges due to the heterogeneity and complexity of their glycan structures. However, advancements in structural biology techniques have enabled researchers to overcome these challenges and obtain detailed insights into the three-dimensional organization of glycoproteins.
X-ray Crystallography: Illuminating the Atomic Details
X-ray crystallography has been a cornerstone technique for determining the atomic structures of proteins, including glycoproteins. By forming well-ordered crystals and subjecting them to X-ray diffraction, researchers can obtain high-resolution structures of glycoproteins. This technique has provided invaluable information about the spatial arrangement of glycans and their interactions with the protein moiety.
Cryo-Electron Microscopy: Visualizing Glycoprotein Assemblies
Cryo-electron microscopy (cryo-EM) has revolutionized the field of structural biology by enabling the visualization of large macromolecular complexes at near-atomic resolution. This technique has been particularly impactful in studying glycoproteins, as it allows for the observation of glycan chains and their interactions with other molecules in their native environment. Through cryo-EM, researchers have elucidated the architectures of glycoprotein assemblies, providing insights into their roles in cell adhesion, viral entry, and immune recognition.
Nuclear Magnetic Resonance Spectroscopy: Probing Glycan Dynamics
Nuclear magnetic resonance (NMR) spectroscopy is a powerful technique for studying the dynamics and interactions of biomolecules in solution. In the case of glycoproteins, NMR spectroscopy has been instrumental in elucidating the conformational flexibility and mobility of glycans, as well as their interactions with proteins and other ligands. By employing advanced NMR techniques, researchers have gained insights into the conformational landscapes of glycoproteins, providing a foundation for understanding their functional diversity and engineering new therapeutic strategies.
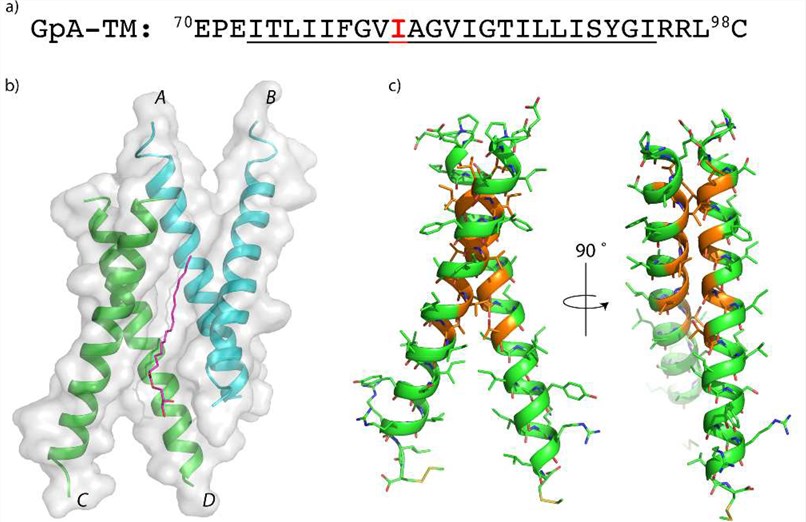 Figure 1. Structure of a Glycophorin A TM peptide crystallized in a monoolein lipidic cubic phase. (Trenker R, et al., 2015)
Figure 1. Structure of a Glycophorin A TM peptide crystallized in a monoolein lipidic cubic phase. (Trenker R, et al., 2015)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| Glycophorin A (GpA) transmembrane-domain dimer (expressed in Escherichia coli) | Homo sapiens | Solution NMR | / | 1AFO |
| Glycophorin A (GpA) transmembrane-domain dimer (expressed in Escherichia coli) | Homo sapiens | Solution NMR | / | 2KPF |
| Glycophorin A (GpA) transmembrane-domain dimer (expressed in Escherichia coli) | Homo sapiens | X-ray diffraction | 2.81 Å | 5EH4 |
| Influenza Hemagglutinin, full length | Influenza A virus | Cryo-EM single particle analysis | 4.20 Å | 6HJR |
| Influenza Hemagglutinin, ectodomain of X-31 Haemagglutinin at pH 8 (expressed in Gallus gallus) | Influenza virus | Cryo-EM single particle analysis | 3.00 Å | 6Y5G |
Table 1. Structural Research of Glycoproteins.
At Creative Biostructure, we are at the forefront of providing comprehensive structural analysis services for glycoproteins. Leveraging our extensive experience and cutting-edge technologies, we offer tailored solutions to investigate the structural intricacies of glycoproteins and their complexes. Our multidisciplinary approach combines X-ray crystallography, cryo-EM, NMR spectroscopy, and advanced computational methods to generate high-resolution structures and unravel the functional implications of glycoproteins. Contact us to discuss your glycoprotein structural analysis needs and embark on a transformative scientific journey.
References
- Trenker R, et al. Crystal structure of the glycophorin A transmembrane dimer in lipidic cubic phase. Journal of the American Chemical Society. 2015, 137(50): 15676-15679.
- Benton D J, et al. Structural transitions in influenza haemagglutinin at membrane fusion pH. Nature. 2020, 583(7814): 150-153.
