Exosomes Loading Drugs by Sonication
Exosomes are a class of extracellular vesicles with a diameter of 30-150nm and a phospholipid bilayer structure that can carry a variety of essential biological information such as proteins, nucleic acids, and lipids. Almost all cells can actively secrete exosomes as natural signaling messengers between cells and play the role of intercellular communication. In recent years, the properties of stability, high biocompatibility, and low immunogenicity have made exosomes an ideal platform for drug delivery. The critical aspect of exosomes as drug carriers is the effective loading of exogenous drugs into exosomes. Currently, sonication has been widely used for exosome drug loading.
Why Use Exosomes as Drug Loaders?
Exosome-based intercellular communication is highly conserved and is widely involved in major pathological/physiological processes including cellular homeostasis, proliferation, cancer development, and cardiovascular diseases. The natural properties of exosomes make them promising drug-delivery vehicles for delivering various chemicals, proteins, nucleic acids, etc. As a new field of modern drug delivery, the advantages of exosomes are mainly as follows:
- Exosomes can be engineered and genetically modified to express different surface receptors.
- Exosomes have specific targeting, targeting specific tissues and organs.
- Low toxicity and low immunogenicity.
- In high biocompatibility, exosomes are easily taken up by various cells.
- Exosomes can cross biological barriers, such as the blood-brain barrier, for intracranial drug delivery.
- Long circulation time, exosomes protect the carried therapeutic molecules and reduce their degradation in the body.
- Exosomes can be used for drug delivery through different routes.
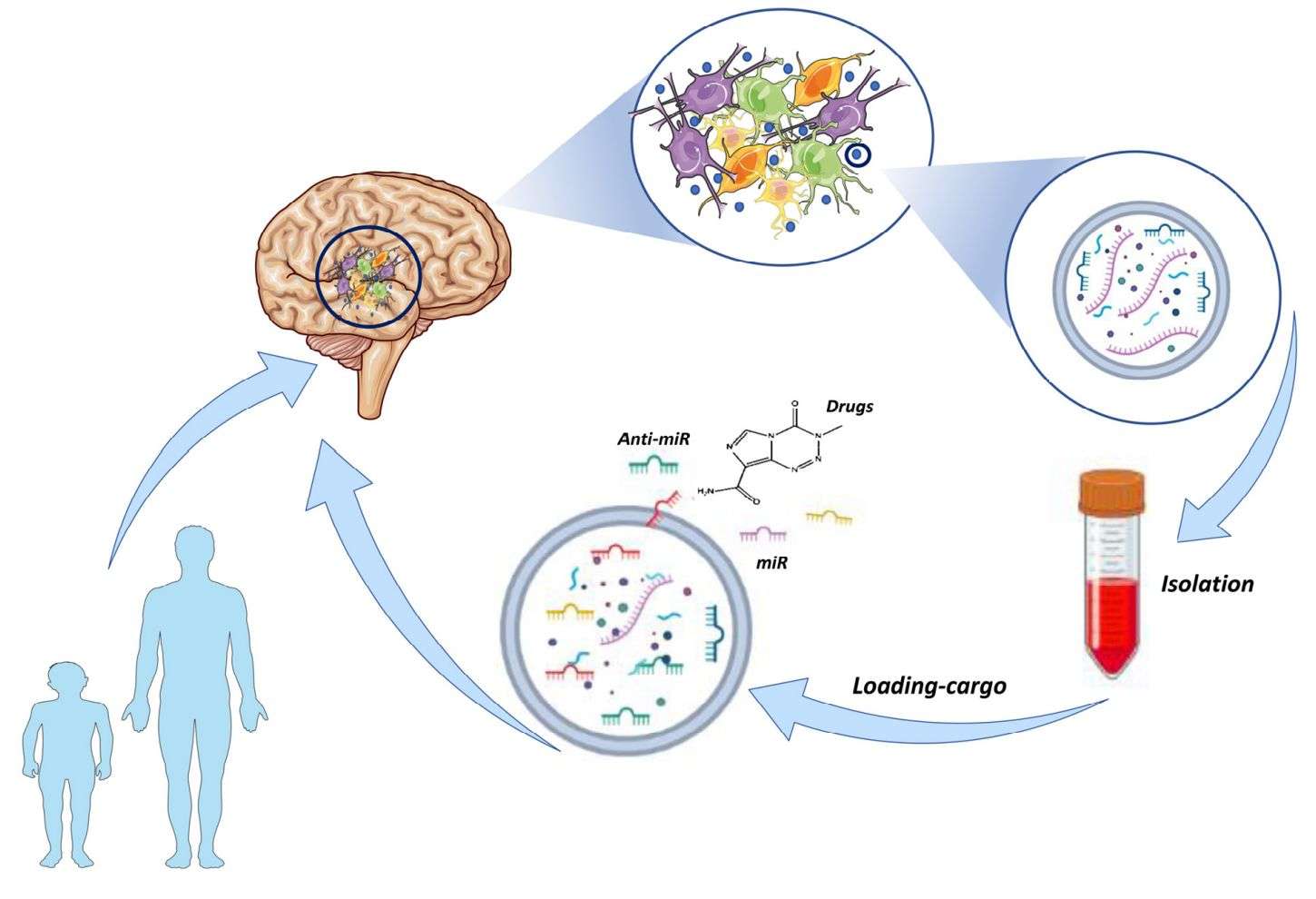 Figure 1. Exosome-based drug delivery therapies. (Galardi A, et al., 2023)
Figure 1. Exosome-based drug delivery therapies. (Galardi A, et al., 2023)
What is the Sonication Method?
The sonication method utilizes a probe sonicator to sonicate exosomes. The mechanical shear of the ultrasound probe alters the integrity of the exosome membrane, creating transient pores that allow the diffusion of small molecules into the exosome. This method is primarily used to load hydrophilic molecules. After sonication, the high rigidity of the exosome membrane is reduced, allowing the drug to be incorporated into the lipid bilayer and improving the loading capacity. In addition, the drug may be attached to the exosome surface. The drug-loaded exosomes prepared by sonication have a non-spherical morphology, and the disruption of exosome integrity may lead to changes in the immune privilege.
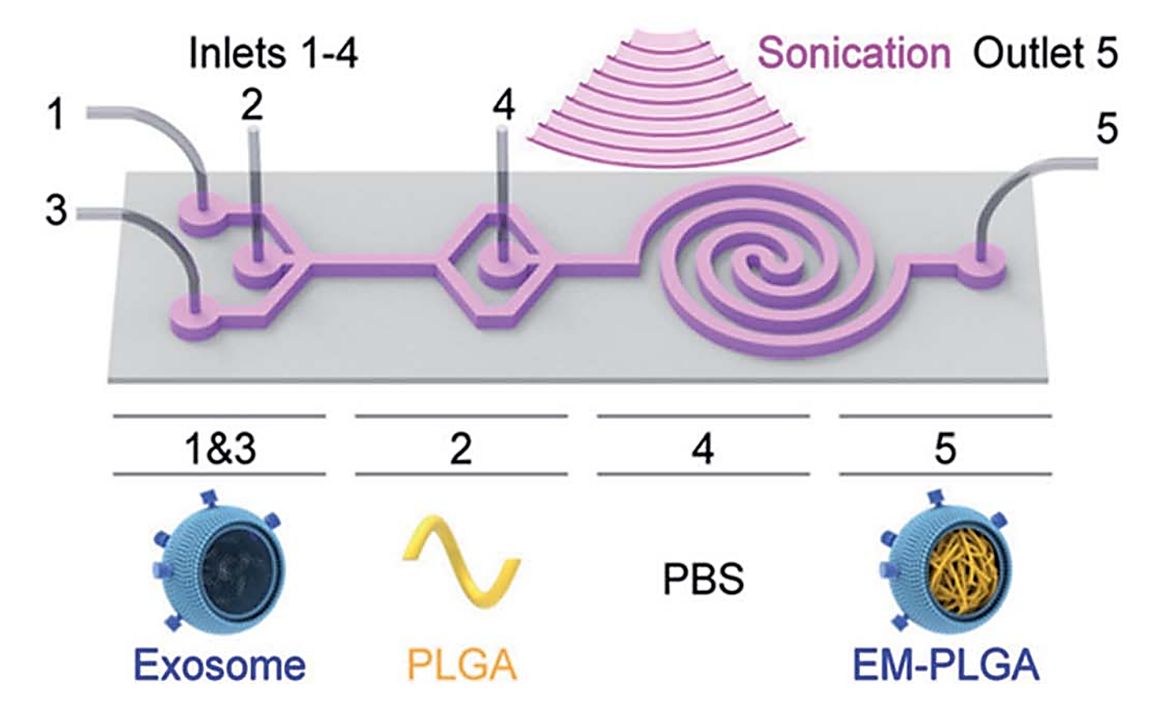 Figure 2. Schematic illustration of the sonication-assisted encapsulation process for PLGA-loaded exosome structure (EM-PLGA). (Lee J, et al., 2022)
Figure 2. Schematic illustration of the sonication-assisted encapsulation process for PLGA-loaded exosome structure (EM-PLGA). (Lee J, et al., 2022)
Sonication has the distinct highlights of increasing the entry of a variety of small nucleic acids into exosomes, and sonicated exosomes retain their ability to transport small RNA cargoes. Sonication also formed fewer aggregates than electroporation. In addition, exosome membranes retain specific adhesion proteins that are particularly essential for interaction with target cells after sonication.
Exosome Drug-loading Sonication Process
1. Raw material preparation: Collect or culture exosomes and drugs.
2. Exosome isolation: Isolate and purify exosomes from samples by ultracentrifugation or other methods.
3. Preparation of exosome-drug mixture.
4. Ultrasonic pretreatment: Ultrasonic treatment of the mixture using an ultrasonic crusher to promote the entry of the drug into the exosome.
5. Second step centrifugation: Ultrasonic centrifugation of the mixture again to remove the free drug.
6. Collection of exosome-drug nanoparticles.
7. Quality control: Characterize the morphology and particle size distribution of exosome-drug nanoparticles by transmission electron microscopy (TEM) and dynamic light scattering (DLS).
8. Follow-up animal experiments or clinical research: Exosome-drug nanoparticles are used in animal experiments or clinical research to evaluate their distribution, targeting, and therapeutic effects in vivo.
Examples of Exosome Drug Loading by Sonication
Hepatocellular carcinoma has a high mortality rate and its treatment requires efficient and specific induction of liver cancer cell death. Researchers loaded iron death inducer (Erastin, Er) and photosensitizer (Rose Bengal, RB) into exosomes and delivered them to tumor tissues by sonication. The research found that the drug-loaded engineered exosomes strongly induced iron death in tumor cells both in vivo and in vitro with significantly reduced liver toxicity. Exosomes will provide a promising strategy for the treatment of a wide range of malignant tumors.
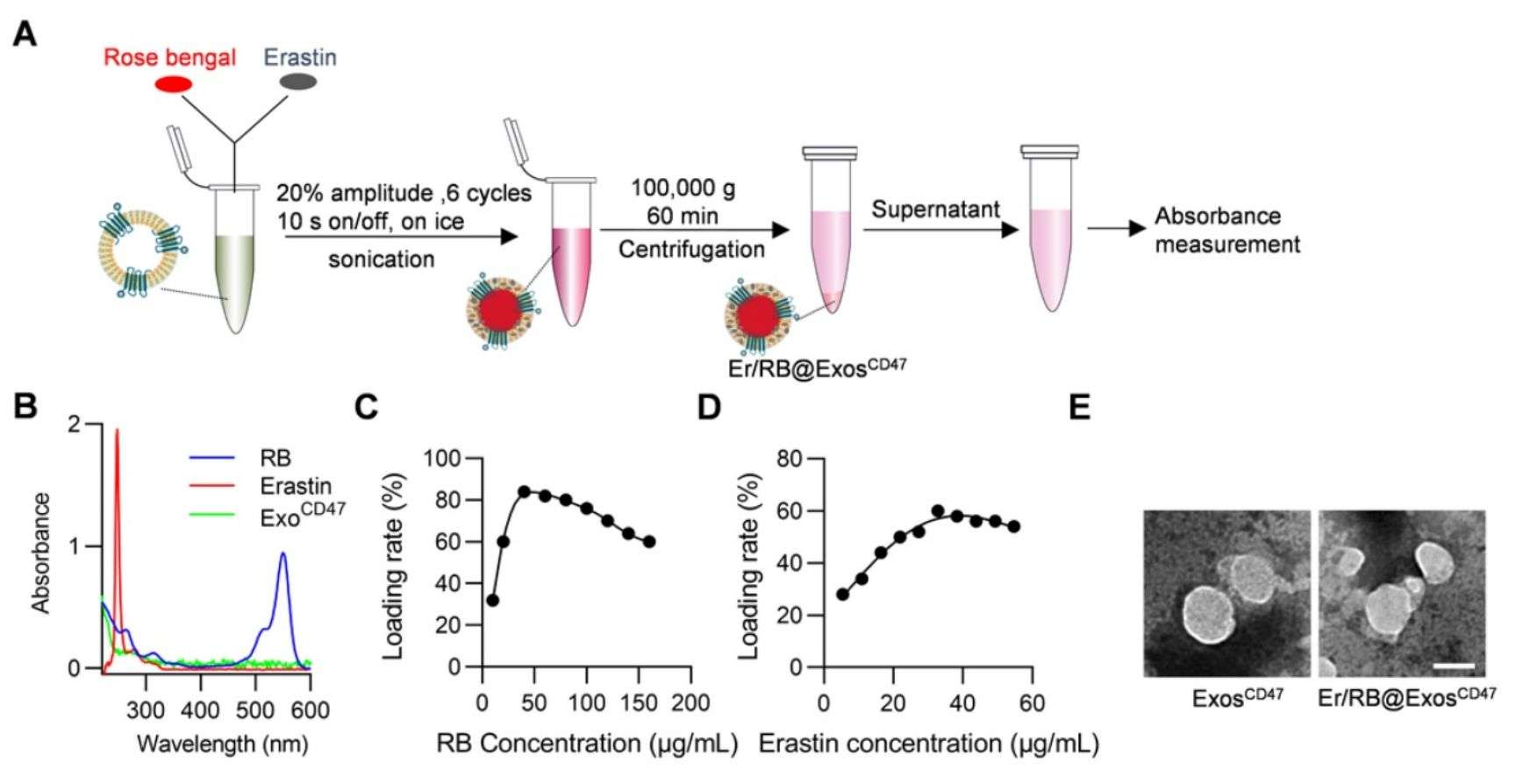 Figure 3. Schematic of loading of Er and RB into exosomes by sonication. (Du J, et al., 2021)
Figure 3. Schematic of loading of Er and RB into exosomes by sonication. (Du J, et al., 2021)
Glioblastoma is one of the deadliest cancers, so new and effective treatments are urgently needed. Single-domain antibodies are one of them, which can target intracellular proteins. To increase its efficiency, delivery systems should be applied. Researchers have researched exosomes as a delivery system for anti-vimentin single-domain antibody Nb79. The research demonstrated that sonication is an appropriate method for loading Nb79 into exosomes. This method can also be translated to other applications, such as targeted delivery systems for other protein-based drugs.
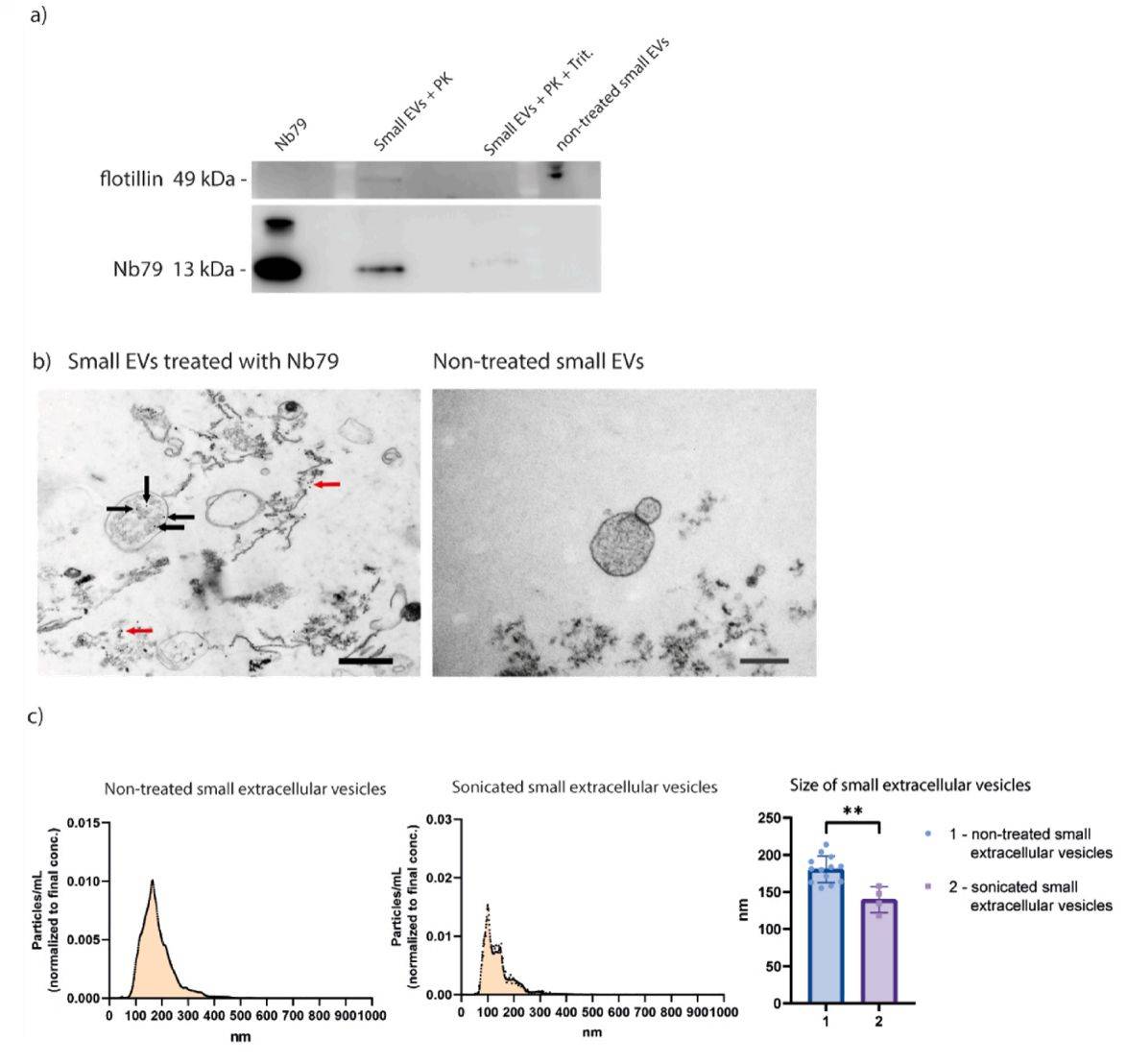 Figure 4. Characterization of exosome loaded with Nb79 using sonication method. (Colja S, et al., 2023)
Figure 4. Characterization of exosome loaded with Nb79 using sonication method. (Colja S, et al., 2023)
Our Products
Creative Biostructure is a specialized biotechnology company offering a range of cutting-edge exosome services with a special focus on exosome drug delivery solutions. Utilizing breakthrough technologies such as sonication, we effectively load therapeutic drugs into exosomes for targeted delivery and improved cancer treatment outcomes.
In addition to our innovative exosome drug-loading services, we offer a full range of exosome products to satisfy clients' requirements.
| Cat No. | Product Name | Source |
| Exo-CM01 | HQExo™ Exosome-B16F10 | Exosome derived from murine melanoma cell line (B16F10 cell line) |
| Exo-CH19 | HQExo™ Exosome-NCI-H975 | Exosome derived from human lung carcinoma (non-small cells) (NCI-H975 cell line) |
| Exo-CH24 | HQExo™ Exosome-SK-BR-3 | Exosome derived from human breast carcinoma cell line (SK-BR-3) |
| Exo-GC07 | HQExo™ Exosome-CD63-EGFP | Exosome derived from human embryonic kidney cell line (HEK293, CD63-EGFP) |
| Exo-GC03 | HQExo™ Exosome-GFP | Exosome derived from HEK293 cell line with GFP loaded |
| Exo-HDBF-05 | HQExo™ Exosome-SDH-Benign Organ Tumor plasma | Exosome derived from Single Donor Human Benign Organ Tumor plasma |
| Exo-HDBF-14 | HQExo™ Exosome-SDH-Bladder Cancer Plasma exosome | Exosome derived from Single Donor Human Bladder Cancer Plasma |
| Exo-PDELN04 | HQExo™ Exosome-Potato | Exosome derived from Potato |
| Exo-PDELN05 | HQExo™ Exosome-Seaberries | Exosome derived from Seaberries |
| Explore All Exosomes Products | ||
Our technical team is dedicated to utilizing the unique properties of exosomes to optimize drug delivery, ensuring that drugs are delivered precisely and efficiently to specific tissues or cells. Please feel free to contact us for more information. Our team of experts is dedicated to providing superior exosome solutions and high-quality exosome products to advance the research of exosomes in drug delivery.
References
- Galardi A, et al. Recent Advancements on the Use of Exosomes as Drug Carriers for the Treatment of Glioblastoma. Life. 2023. 13(4): 964.
- Lee J, et al. Exosome-based drug delivery systems and their therapeutic applications. RSC Adv. 2022. 12(29): 18475-18492.
- Du J, et al. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. 2021. 11(17): 8185-8196.
- Colja S, et al. Sonication is a suitable method for loading nanobody into glioblastoma small extracellular vesicles. Heliyon. 2023. 9(5): e15674.
- Zeng H, et al. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells. 2023. 12(10): 1416.
- Xi XM, et al. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021. 76(2): 61-67.
- Zhang Y, et al. Exosomes as Anticancer Drug Delivery Vehicles: Prospects and Challenges. Front Biosci (Landmark Ed). 2022. 27(10): 293.
