Structural Research of Bacteriophage MS2
Bacteriophage MS2, a member of the Levivirus genus in the Leviviridae family, is a small, positive-sense, single-stranded ribonucleic acid (RNA) phage that infects hosts by attaching to bacterial hyphae. It is small and contains mature proteins, shell proteins, and genomic RNA. it also has one of the smallest known genomes, encoding four proteins. In practice, structural components of MS2 have been used to detect RNA in living cells. The virus is also being researched for its potential use in drug delivery, tumor imaging, and light capture.
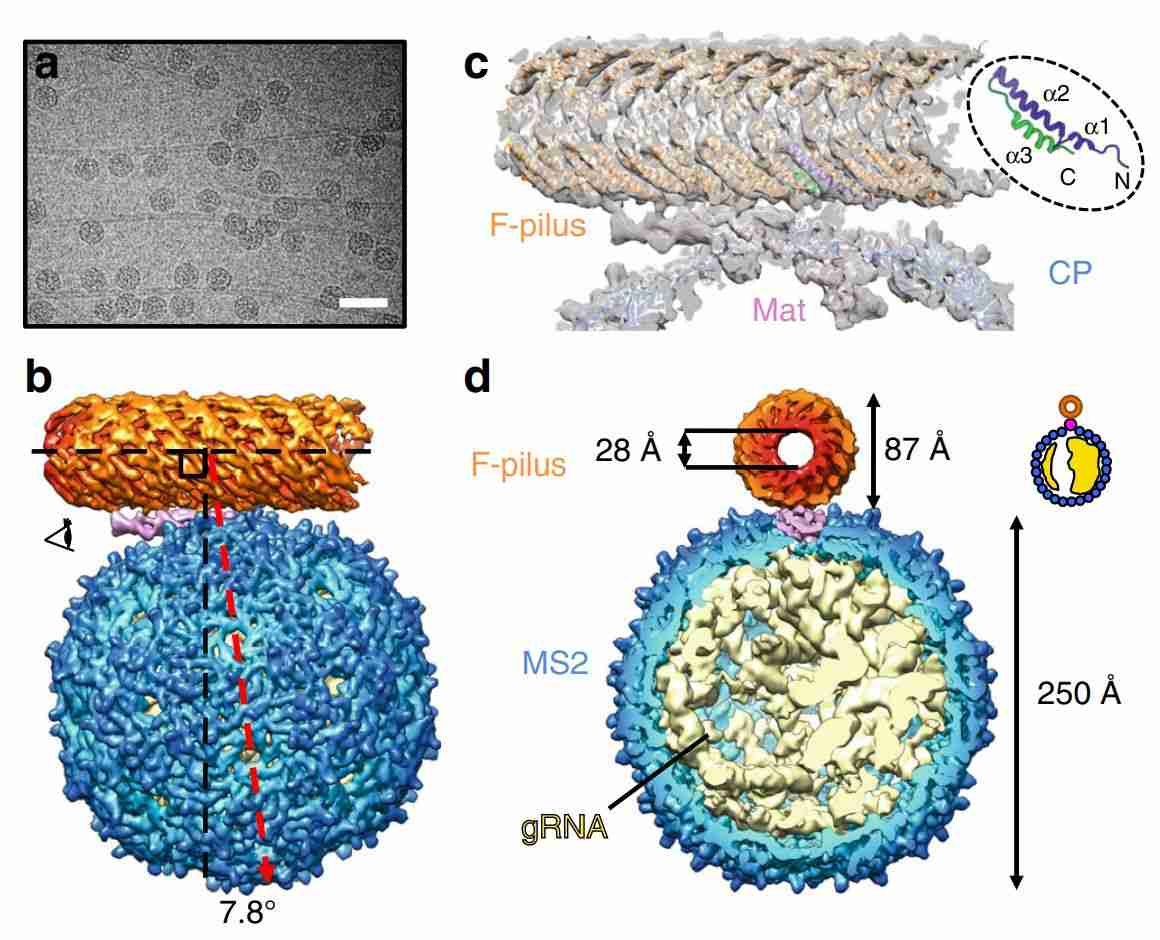 Figure 1. The overall architecture of the MS2/F-pilus complex. (Meng R, et al., 2019)
Figure 1. The overall architecture of the MS2/F-pilus complex. (Meng R, et al., 2019)
Structural Feature of Bacteriophage MS2
The genome of phage MS2 spans 3,569 nucleotides and consists of a mature protein monomer and 89 capsid dimers. MS2 infects Escherichia coli by interacting with mature proteins and bacterial F-pili, delivering its genome and mature proteins (MPs) into host cells. In vivo, the capsid of MS2 particles has pseudo icosahedral symmetry (T=3) and coalesces around the genome during assembly. Single-stranded RNA within the virus forms secondary structures that interact directly with capsid proteins as packaging signals. Of these signals, the stem-loop structure has been most extensively researched. During assembly, the RNA specifies three quasi-equivalent conformations (A, B, and C) of the phage protein, forming the infection assembly unit (IAU). These subunits interact to generate two different types of dimers: the asymmetric A/B dimer, which extends from the fivefold to the threefold axis, and the symmetric C/C dimer, which lies on the bipartite axis.
Research Progress on Bacteriophage MS2
Previous research used X-ray crystallography to determine atomic models of the MS2 capsid, capsid protein dimers, and RNA TR hairpin loops bound to the CP, revealing sequence-specific RNA-protein interactions. Subsequently, icosahedral cryo-electron microscopy (cryo-EM) of MS2 viral particles, including the genome and AP, is reconstructed at 8.7 Å. The results of this study are summarized below. In addition, the researchers demonstrated single-particle cryo-EM structures of different conformations of the MS2/F-pilus complex with resolutions ranging from 5.6 to 7.3 Å, revealing the interaction network at the phage-hair interface. In recent years, research has revealed that MS2 VLP is a 27nm spherical RNA virus-like particle composed of 180 identical capsid protein subunits. Due to its small size and ability to encapsulate different nucleic acids, proteins, and other small molecules, MS2 VLP is considered an appropriate vector for delivery and target tracking.
Research on the structure of MS2 can provide insight into the mechanism of infection by this virus. It can help to elucidate the molecular events involved in viral attachment and entry, leading to the development of strategies to prevent or disrupt MS2 infection. In addition, further studies of the secondary structure elements of single-stranded RNA and their interactions with coat proteins could enhance the understanding of genome packaging, replication, and transcription processes, with implications for the design of gene delivery systems or research on the assembly of other related viruses.
As experts in structural biology, Creative Biostructure provides high-quality virus-like particles (VLPs) products for structural research on bacteriophage MS2 and many other types of viruses. We offer clients a safe and controlled experimental platform that contributes to basic research, drug discovery, and diagnostics in the field of virology.
| Cat No. | Product Name | Virus Family | Source | Composition |
| CBS-V543 | Bacteriophage MS2 VLP (coat protein Proteins) | Leviviridae | E. coli recombinant | coat protein |
| CBS-V544 | Bacteriophage MS2 VLP (coat protein Proteins) | Leviviridae | Yeast recombinant | coat protein |
| CBS-V545 | Bacteriophage MS2 VLP (coat protein Proteins) | Leviviridae | Mammalian cell recombinant | coat protein |
| Explore All Bacteriophage MS2 VLP Products | ||||
In addition, Creative Biostructure provides structural research of viruses, including X-ray crystallography and cryo-electron microscopy (cryo-EM). With cutting-edge equipment and a dedicated team of professionals, we are committed to providing our clients with high-quality research services on the structural biology of viruses.
If you are interested in exploring the structure of bacteriophage MS2 and would like more information about the services we offer, please contact us. Our team is always available to discuss your research requirements and provide the most effective solution for your project.
References
- Meng R, et al. Structural basis for the adsorption of a single-stranded RNA bacteriophage. Nat Commun. 2019. 10(1): 3130.
- Hashemi K, et al. Author Correction: Optimizing the synthesis and purification of MS2 virus-like particles. Sci Rep. 2022. 12(1): 8681.
- de Martín Garrido N, et al. Bacteriophage MS2 displays unreported capsid variability assembling T = 4 and mixed capsids. Mol Microbiol. 2020. 113(1): 143-152.
- Koning RI, et al. Asymmetric cryo-EM reconstruction of phage MS2 reveals genome structure in situ. Nat Commun. 2016. 7: 12524.
