Structural Research of Wntless (WLS) Transporters
Research has identified Wntless (WLS) as an evolutionarily conserved multiple transmembrane protein essential for Wnt protein secretion. Wnt proteins are secreted glycoproteins integral to embryonic development and adult tissue homeostasis. In recent years, due to the rapid development of crystallography and cryo-electron microscopy, researchers have revealed the cellular mechanisms responsible for Wnt secretion through structural analyses. The molecular mechanisms that regulate Wnt secretion may help intervene in Wnt production and signal transduction activity in certain cancers.
The role of WLS in Wnt secretion
After lipid modification by the acyltransferase Porcupine, Wnt proteins bind WLS for transport and secretion. WLS regulates the exocytosis of Wnt from the trans-Golgi network (TGN) to the cell membrane. Without WLS, Wnt proteins are retained in their expressing cells. Once Wnt is exocytosed, WLS is internalized by lattice protein-mediated endocytosis and transported to the TGN via the retrotransposon complex. Without reverse transcriptase, Wls is eventually degraded in the lysosome.
Overall structural analysis of WLS
In recent years, researchers have used cryo-electron microscopy to determine the molecular structure of WLS-bound palmitoylated human WNT8A. This method allows the structure of both proteins to be studied in a near-natural state, without the interference of staining or fixation required for conventional electron microscopy. The structures show that WLS consists of two parts, an integral membrane structural domain consisting of eight TM helices, with the N- and C-termini located on the cytosolic side of the membrane, and a globular inner lumenal structural domain (WLS-LD) located between TM1 and TM2, which is required for Wnt binding. The WLS-LD consists of eight antiparallel β-sheets that resemble a β-sandwich. WLS has a conserved lectin-mediated endocytosis motif YXXΦ at residue Tyr425, where Φ is a hydrophobic residue.
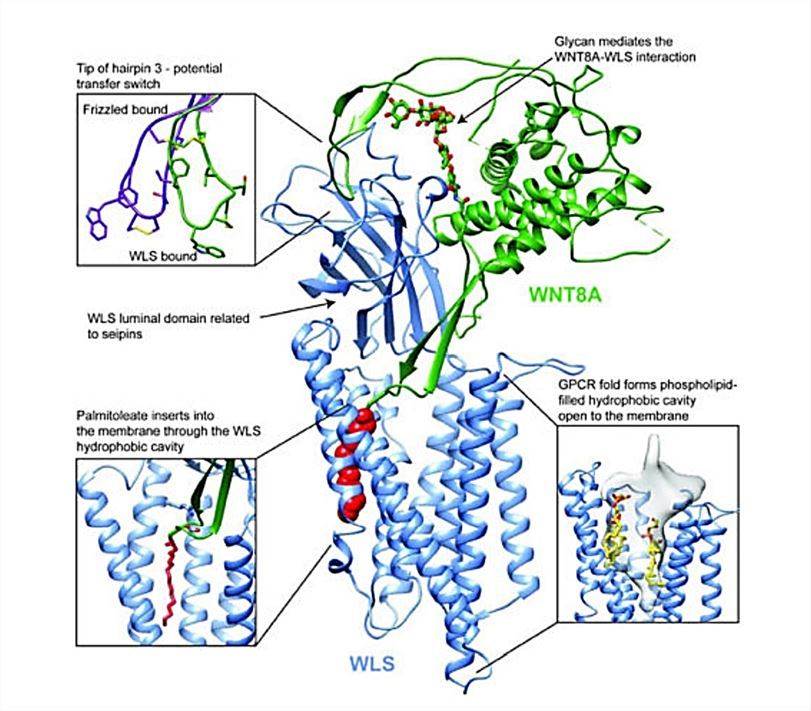 Figure 1. The overall structure of WNT8A and WLS complexes. (Nygaard R, et al., 2021)
Figure 1. The overall structure of WNT8A and WLS complexes. (Nygaard R, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| WLS | Homo sapiens | Cryo-EM single particle analysis | 3.84 Å | 8TZS |
| Wnt7a bound to WLS and RECK | Homo sapiens | Cryo-EM single particle analysis | 3.23 Å | 8TZP |
| Wnt7a bound to WLS and CALR | Homo sapiens | Cryo-EM single particle analysis | 3.1 Å | 8TZO |
| Wnt3a bound to WLS and CALR | Homo sapiens | Cryo-EM single particle analysis | 3.5 Å | 8TZR |
| WLS in complex with WNT8A | Homo sapiens | Cryo-EM single particle analysis | 3.19 Å | 7KC4 |
| Wntless in complex with Wnt3a | Homo sapiens | Cryo-EM single particle analysis | 2.2 Å | 7DRT |
Table 1. Structural research of the wntless (WLS) transporters.
Creative Biostructure understands the importance of obtaining high-quality, reliable structural information for protein function research and drug discovery. Our team of experienced scientists, cutting-edge equipment, and proven track record of success make us your ideal partner in structural biology. Our scientists help clients explore the crystal structure of wntless (WLS) transporters through X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy to reveal the underlying cellular mechanisms by which WLS mediates WNT secretion.
We can also provide professional guidance to ensure the success of your project. If you want to learn more about our services and how we can help you achieve your goals, please do not hesitate to contact us.
References
- Nygaard R, et al. Structural Basis of WLS/Evi-Mediated Wnt Transport and Secretion. Cell. 2021. 184(1): 194-206. e14.
- Zhong Q, et al. Cryo-EM structure of human Wntless in complex with Wnt3a. Nat Commun. 2021. 12(1): 4541.
- Zhang P, et al. Dysfunction of Wntless triggers the retrograde Golgi-to-ER transport of Wingless and induces ER stress. Sci Rep. 2016. 6: 19418.