What is XRF (X-ray Fluorescence) and How Does it Work?
X-ray fluorescence (XRF) is an important analytical technique in structural biology, aiding in the identification and quantification of elements within biological samples. What makes XRF stand out is its non-destructive nature, making it useful across many fields, including geology, archaeology, environmental science, and biology. In structural biology, XRF is particularly valued for uncovering elemental compositions and distributions. This provides critical insights into cellular and molecular structures. Its precision in detecting and quantifying trace metals and other elements is essential for studying biomolecules, metalloproteins, and mineralized tissues.
Principle of X-ray Fluorescence
The principle of XRF lies in the interaction between high-energy X-rays and the atoms in a sample. When a sample is bombarded with primary X-rays, it excites the atoms by dislodging inner-shell electrons, creating electron vacancies. Outer-shell electrons subsequently fill these vacancies, emitting secondary or fluorescent X-rays. Each element emits X-rays at characteristic energy levels, creating a spectral fingerprint unique to that element. This phenomenon enables XRF to detect and quantify elements based on their specific energy emissions.
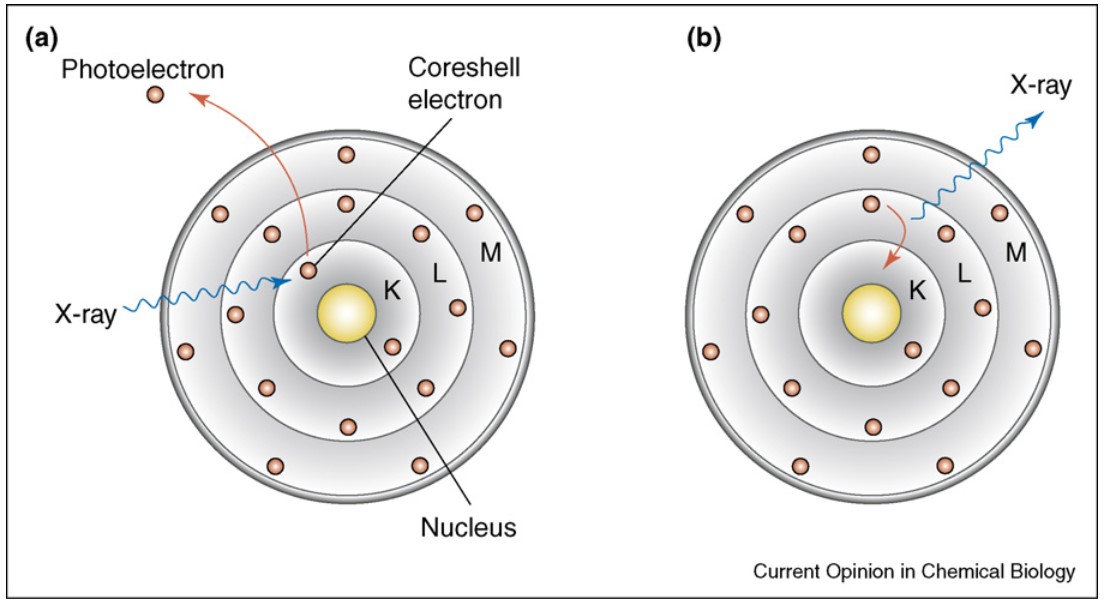 Figure 1: Bohr atom model illustrating the basic principle of X-ray fluorescence. (a) X-ray excitation leads to ejection of a core-shell electron from the atom. (b) The generated vacancy is filled through a higher-shell electron, a process that results in emission of a photon whose energy is equal to the difference in binding energies of the two shells involved in the transition. (Fahrni, 2007)
Figure 1: Bohr atom model illustrating the basic principle of X-ray fluorescence. (a) X-ray excitation leads to ejection of a core-shell electron from the atom. (b) The generated vacancy is filled through a higher-shell electron, a process that results in emission of a photon whose energy is equal to the difference in binding energies of the two shells involved in the transition. (Fahrni, 2007)
In practical applications, XRF instruments consist of three main components: an X-ray source, a sample holder, and a detector. The X-ray source, often an X-ray tube or radioactive source, emits primary X-rays that target the sample. The detector then measures the secondary X-rays emitted from the sample. Advanced detectors like silicon drift detectors (SDDs) enhance this process. They offer high-resolution detection and are especially useful for analyzing complex samples. The data collected creates an XRF spectrum. This spectrum reveals the elements present in the sample and their concentrations, based on the energies and intensities of the peaks.
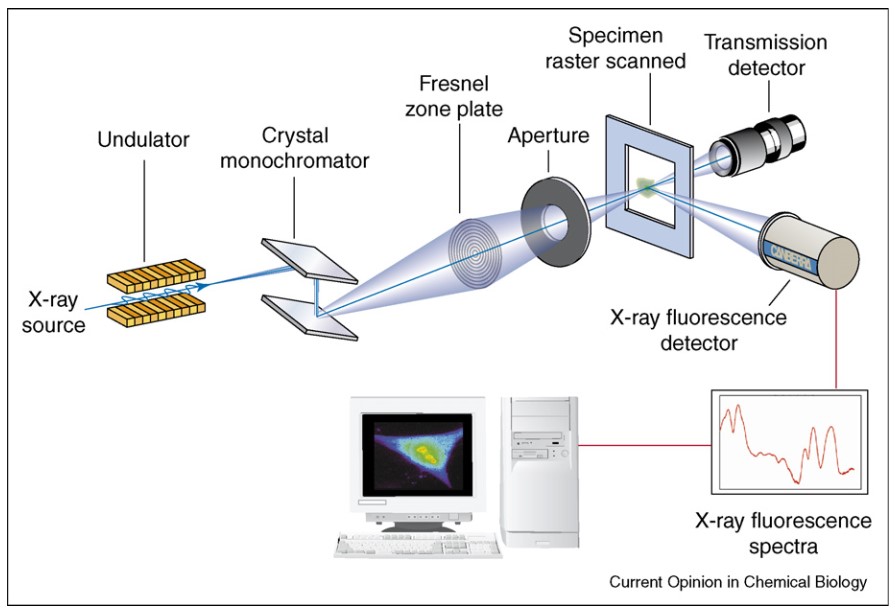 Figure 2: Schematic diagram illustrating the components of an X-ray fluorescence microscope. A crystal monochromator is used to select the energy of the incident X-ray beam, which is focused with a Fresnel zone plate on the specimen. The emitted X-rays are collected with an energy dispersive detector, thus allowing for simultaneous multi-element analysis. Raster-scanning across the specimen area yields then quantitative elemental maps, as illustrated on the computer monitor. The purpose of the transmission detector is to help orient the sample on the scanning stage. (Fahrni, 2007)
Figure 2: Schematic diagram illustrating the components of an X-ray fluorescence microscope. A crystal monochromator is used to select the energy of the incident X-ray beam, which is focused with a Fresnel zone plate on the specimen. The emitted X-rays are collected with an energy dispersive detector, thus allowing for simultaneous multi-element analysis. Raster-scanning across the specimen area yields then quantitative elemental maps, as illustrated on the computer monitor. The purpose of the transmission detector is to help orient the sample on the scanning stage. (Fahrni, 2007)
Interpretation of XRF Spectra
Interpreting XRF spectra involves analyzing the energy peaks corresponding to different elements and their intensities. Each element has a distinct peak at a specific energy level, making it possible to identify and quantify multiple elements within a sample simultaneously. The peak height or intensity reflects the element's abundance, enabling quantitative analysis.
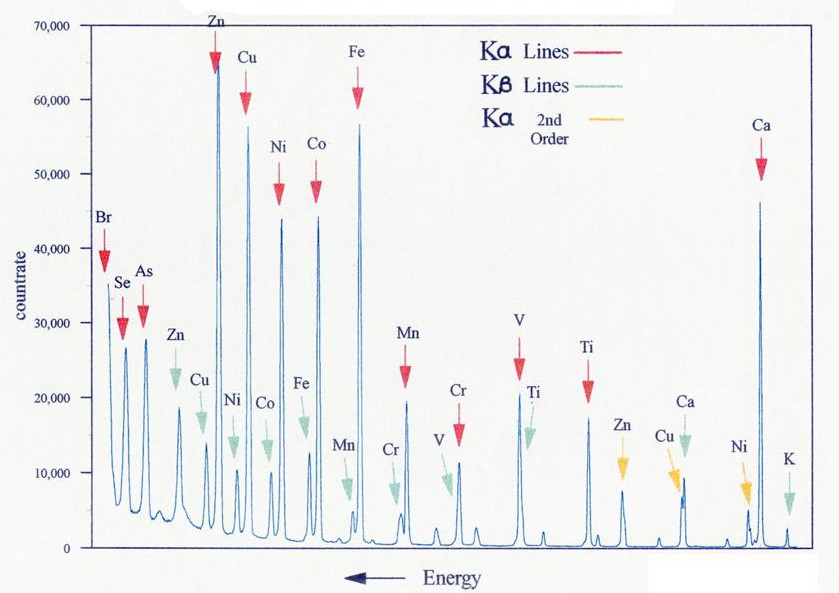 Figure 3: Typical wavelength dispersive XRF spectrum.
Figure 3: Typical wavelength dispersive XRF spectrum.
In biological samples, elements such as calcium, potassium, iron, and zinc are often of particular interest due to their roles in structural and metabolic functions. However, interpreting XRF spectra in biological contexts poses unique challenges due to the complexity of organic matrices and potential interference from similar energy emissions. To overcome these, advanced analytical software and calibration standards are used to enhance accuracy and reliability. Additionally, techniques like micro-XRF, which focuses the X-ray beam on specific regions of a sample, provide detailed spatial mapping of elements, facilitating insights into the distribution of essential metals within cells or tissues.
Applications of XRF in Structural Biology
Elemental Mapping in Biomolecules and Cells
The elemental analysis of biomolecules is an important application of XRF, especially metalloproteins, which are made of metals and cofactors for their activity. By mapping the elemental composition of these proteins, researchers can understand the structure-function relationships within these molecules. For example, iron in hemoglobin or copper in cytochrome c oxidase is analyzed to know what roles they play in oxygen transport and cellular respiration. Micro-XRF has also helped to probe trace elements in cells, allowing for elements such as zinc in enzymes and iron in the metabolism.
Analysis of Mineralized Tissues
Often studied in structural biology are tissues with mineralized materials—bones and teeth for example, which are made of calcium, phosphorus and other trace elements. These tissues can be analyzed by non-destructive analysis using XRF that gives quantitative information about the elemental content. By examining changes in elemental ratio, researchers can determine bone density, evaluate osteoporosis, and assess the influence of mineral deficiency on bones. The mapping of elemental detail with XRF underlies research on bone expansion, mineralization and the effects of aging or disease on bone tissue.
Synchrotron-based XRF for High-Resolution Studies
Synchrotron facilities provide a powerful XRF technique through highly focused X-ray beams, enabling ultra-high-resolution elemental mapping of biological samples. Synchrotron-based XRF has been pivotal in structural biology, offering insights into trace elements and their distribution within protein complexes, cells, and tissues. This high-resolution imaging enables researchers to study nanometer-scale structures, essential for understanding complex molecular interactions in proteins and cellular components.
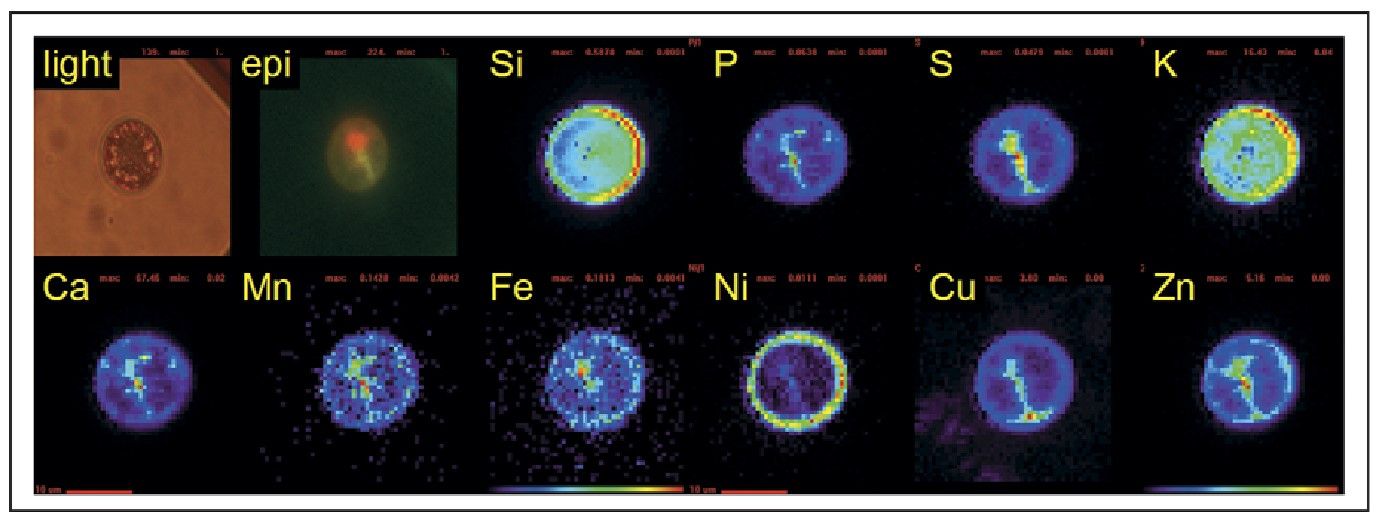 Figure 4: Light and epifluorescence micrographs, and SXRF false-color element maps of a centric diatom collected from the Southern Ocean. Si and K map onto the frustule of the cell, whereas P, S, Ca, Mn, Fe, Cu and Zn appear to be associated with the cytoplasm of the cell (indicated by the green epifluorescence). Fe is most highly concentrated in the chloroplast (region of red epifluorescence), whereas Zn is colocalized with P (likely to be the cell's nucleus). Ni is found on the outer membranes or frustule of the cell. Red scale bars 10 mm. (Fahrni, 2007)
Figure 4: Light and epifluorescence micrographs, and SXRF false-color element maps of a centric diatom collected from the Southern Ocean. Si and K map onto the frustule of the cell, whereas P, S, Ca, Mn, Fe, Cu and Zn appear to be associated with the cytoplasm of the cell (indicated by the green epifluorescence). Fe is most highly concentrated in the chloroplast (region of red epifluorescence), whereas Zn is colocalized with P (likely to be the cell's nucleus). Ni is found on the outer membranes or frustule of the cell. Red scale bars 10 mm. (Fahrni, 2007)
In summary, X-ray fluorescence (XRF) has revolutionized elemental analysis in structural biology by offering a non-destructive, highly precise method for identifying and quantifying elements within biological samples. Its applications span from mapping elemental compositions in biomolecules to analyzing trace metals in cells and tissues, making it a valuable tool in biological research and industrial applications.
At Creative Biostructure, we provide a full range of biological structure assay services, including X-ray, Nuclear Magnetic Resonance (NMR), Cryo-Electron Microscopy (cryo-EM), and more. Equipped with state-of-the-art facilities, we have developed a seamless pipeline that spans every stage, from construct design to final structure determination. Contact us today for the perfect solution!
References
- Fahrni CJ. Biological applications of X-ray fluorescence microscopy: exploring the subcellular topography and speciation of transition metals. Current Opinion in Chemical Biology. 2007;11(2):121-127.
- Gholizadeh A, Coblinski JA, Saberioon M, et al. Vis–NIR and XRF data fusion and feature selection to estimate potentially toxic elements in soil. Sensors. 2021;21(7):2386.
- Pushie MJ, Pickering IJ, Korbas M, Hackett MJ, George GN. Elemental and chemically specific x-ray fluorescence imaging of biological systems. Chem Rev. 2014;114(17):8499-8541.
- Tavares TR, De Almeida E, Junior CRP, Guerrero A, Fiorio PR, De Carvalho HWP. Analysis of total soil nutrient content with x-ray fluorescence spectroscopy (XRF): assessing different predictive modeling strategies and auxiliary variables. AgriEngineering. 2023;5(2):680-697.