Exosome Research in Human Head and Neck Cancer
Head and neck cancer (HNC) is a tumor originating from the tissues or organs of the head and neck. The most common type is head and neck squamous cell carcinoma (HNSCC), which accounts for more than 90% of HNC. The HNC is associated with a high recurrence and metastasis rate, poor patient survival, and a largely unresolved pathology. Currently, therapies for HNC include surgery, radiotherapy, immunotherapy, and targeted therapy. However, despite therapeutic advances, the recurrence of HNC remains a major clinical challenge. Therefore, the discovery of cutting-edge, non-invasive tumor diagnostic and prognostic markers, as well as new therapeutic approaches, is crucial for cancer management.
Exosomes are extracellular vesicles secreted by a variety of cells, 30-150 nm in diameter, that mediate the transport of various molecules (e.g., proteins, nucleic acids, and lipids) between cells throughout the body. During cancer progression, exosomes can deliver cargo to recipient cells as a signal of cancer invasion and metastasis. There is growing evidence of an association between exosomes and HNC aggressiveness.
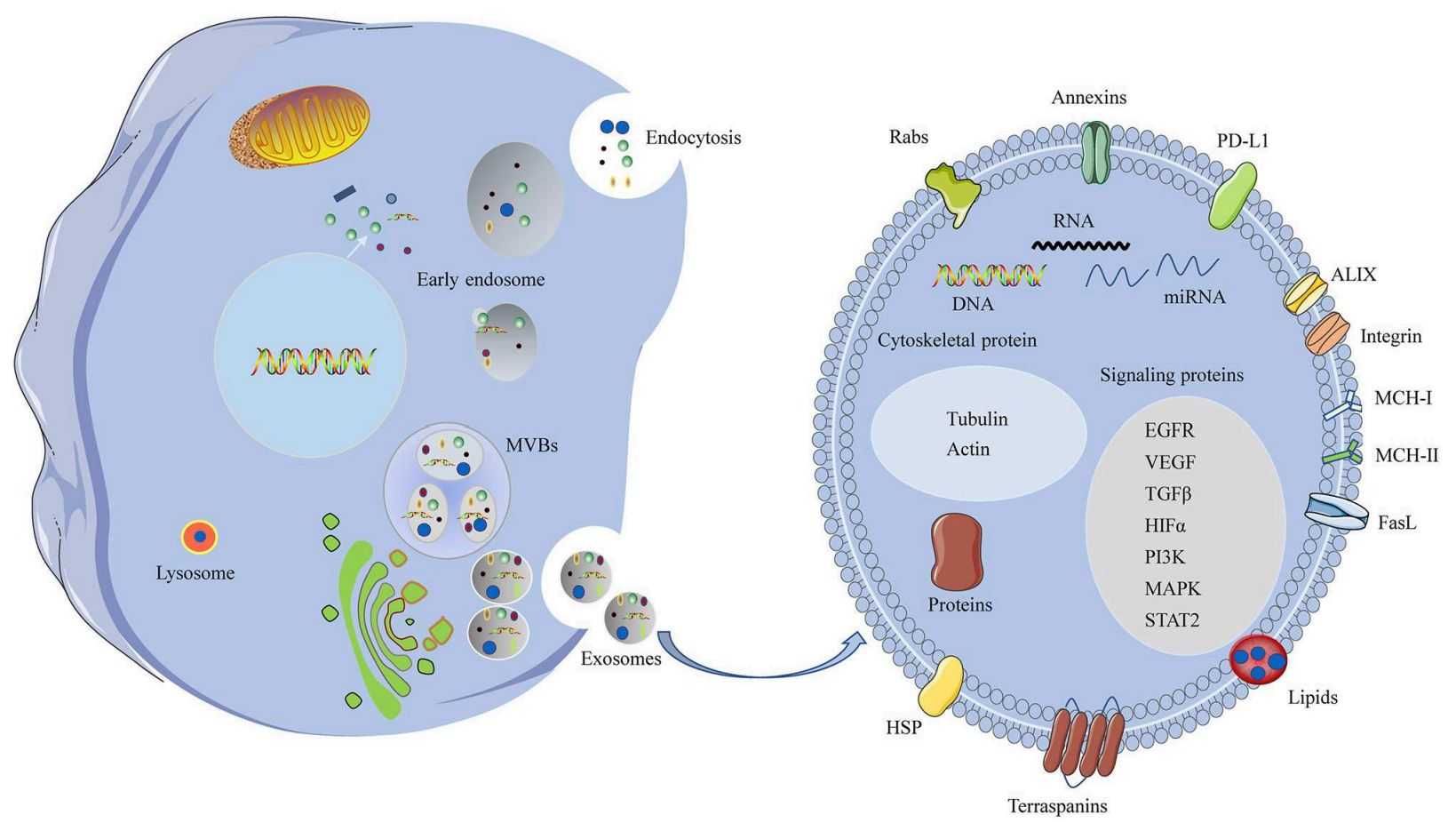 Figure 1. A schematic of biogenesis and components of the HNC exosomes. (Xiao C, et al., 2019)
Figure 1. A schematic of biogenesis and components of the HNC exosomes. (Xiao C, et al., 2019)
The Role of Exosomes in HNC
Exosomes have been reported to influence tumorigenesis, proliferation, migration, and drug resistance, and the role of exosomes in tumor progression is closely related to genetics, tumor type, and cancer stage.
- Exosomes Influence HNC Growth
Angiogenesis is critical for tumor cell proliferation. Research has found that HNSCC-derived exosomes containing TGF-β promote angiogenesis. In addition, CAF-derived exosomes containing miR-3188 affected the proliferation of HNSCC cells. In researching salivary exosomal miRNA as a biomarker for oral squamous cell carcinoma (OSCC), the exogenous exosome miR-24-3p was found to increase the proliferation of recipient malignant cells by targeting the PER1 protein.
- Exosomes Regulate the HNC Microenvironment
The suppressive capacity of immune cells in the tumor microenvironment (TME) is critical for the regulation of anti-tumor immune responses. Researchers have found by mass spectrometry analysis that HNC cells can induce a suppressive phenotype in human CD8+ T cells by releasing TDEs. The immunomodulatory protein galactose lectin-1 in exosomes plays a critical role in inducing this suppressive phenotype.
- Exosomes Promote HNC Drug Resistance
Resistance of HNC to conventional chemotherapeutic agents remains one of the biggest causes of poor treatment response. Exosomes contribute to tumor resistance by sequestering and effluxing cytotoxic drugs, shielding cells from the effects of drugs, and preventing their intracellular accumulation. Exosomes can also contribute to external treatment resistance by mediating intercellular communication and delivering miRNA, mRNA, DNA, or proteins.
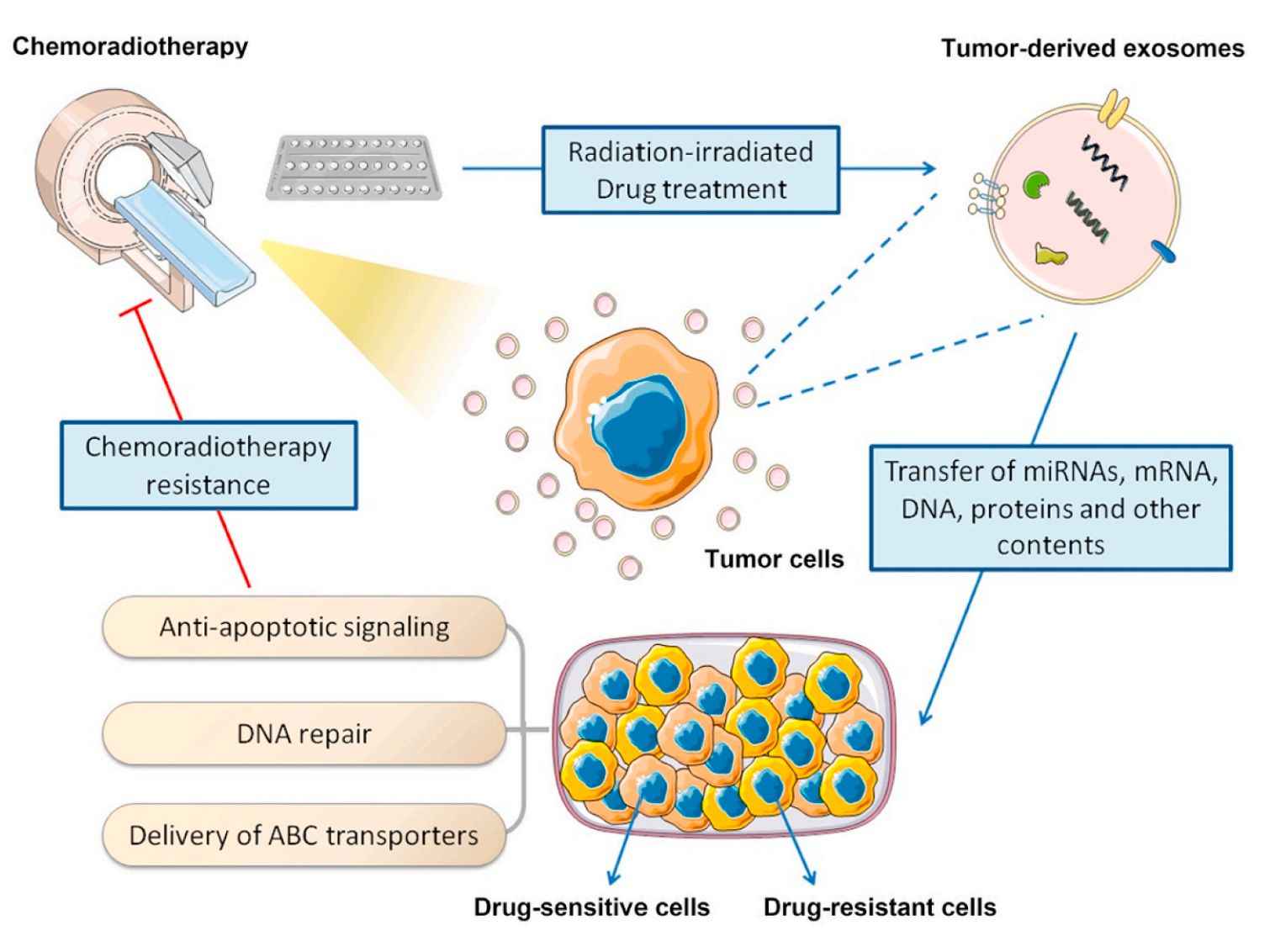 Figure 2. Exosome-induced chemoradiotherapy resistance in HNC. (Cao J, et al., 2020)
Figure 2. Exosome-induced chemoradiotherapy resistance in HNC. (Cao J, et al., 2020)
- Exosomes Regulate Immune Escape and Suppression
Research has shown that exosomes induce phenotypic changes in immune cell populations, thereby triggering immune escape from cancer cells. Impaired maturation and migration of mono-DC was observed in dendritic cells (mono-DC) treated with exosomes from oropharyngeal squamous cell carcinoma (OPHSCC). Gene expression profiles of treated mDC further revealed disruption of immune response by miRNA targeting within exosomes.
- Exosomes are Involved in HNC Invasion and Metastasis
Epithelial-mesenchymal transition (EMT) is an important feature of tumor progression. Exosome-mediated intercellular communication contributes significantly to the EMT process. Research has shown that CD44v3+ tumor cell-derived exosomes contain higher levels of immunosuppressive proteins than healthy controls. The relative fluorescence intensity of these markers correlated with higher disease stage and lymph node metastasis.
As cell-secreted membrane-bound nanoparticles, exosomes deliver a variety of bioactive molecules and play a critical role in HNC cell-cell/extracellular matrix communication. To help clients explore the action mechanism of exosomes in HNC progression to find highly sensitive and specific biomarkers as well as to develop innovative therapeutic strategies, we offer high-quality exosome products.
| Cat No. | Product Name | Source |
| Exo-HDBF-17 | HQExo™ Exosome-SDH-Head and Neck Cancer Plasma exosome | Exosome derived from Single Donor Human Head and Neck Cancer Plasma |
| Explore All Exosomes Isolated from Human Head and Neck Cancer Plasma | ||
Exosomes as Potential Biomarkers for HNC
Developing effective cancer treatment strategies remains challenging. In the last decade, exosomes have been shown to contain the same bioactive substances as donor cells, emerging as potential biomarkers for HNC diagnosis. Exosomal miRNAs are common in HNSCC liquid biopsies. It has been reported that miRNAs (e.g., miR-31, miR-142, miR-186,) can constitute potential prognostic biomarkers for HNSCC. Research has also confirmed that tumor cell-derived exosomes secrete heat shock protein (HSP), which is significantly higher in HNSCC patients and could be used as a biomarker for cancer metastasis. Since CD44v3 overexpression is associated with poorer prognosis, this glycoprotein is a potential biomarker for HNSCC. ANXA1 has been shown to play a tumor suppressor role in HNSCC, and reduced levels of ANXA may also be an important prognostic biomarker. In addition, EGFR-related exosomal miRNAs are valuable in monitoring HNSCC progression.
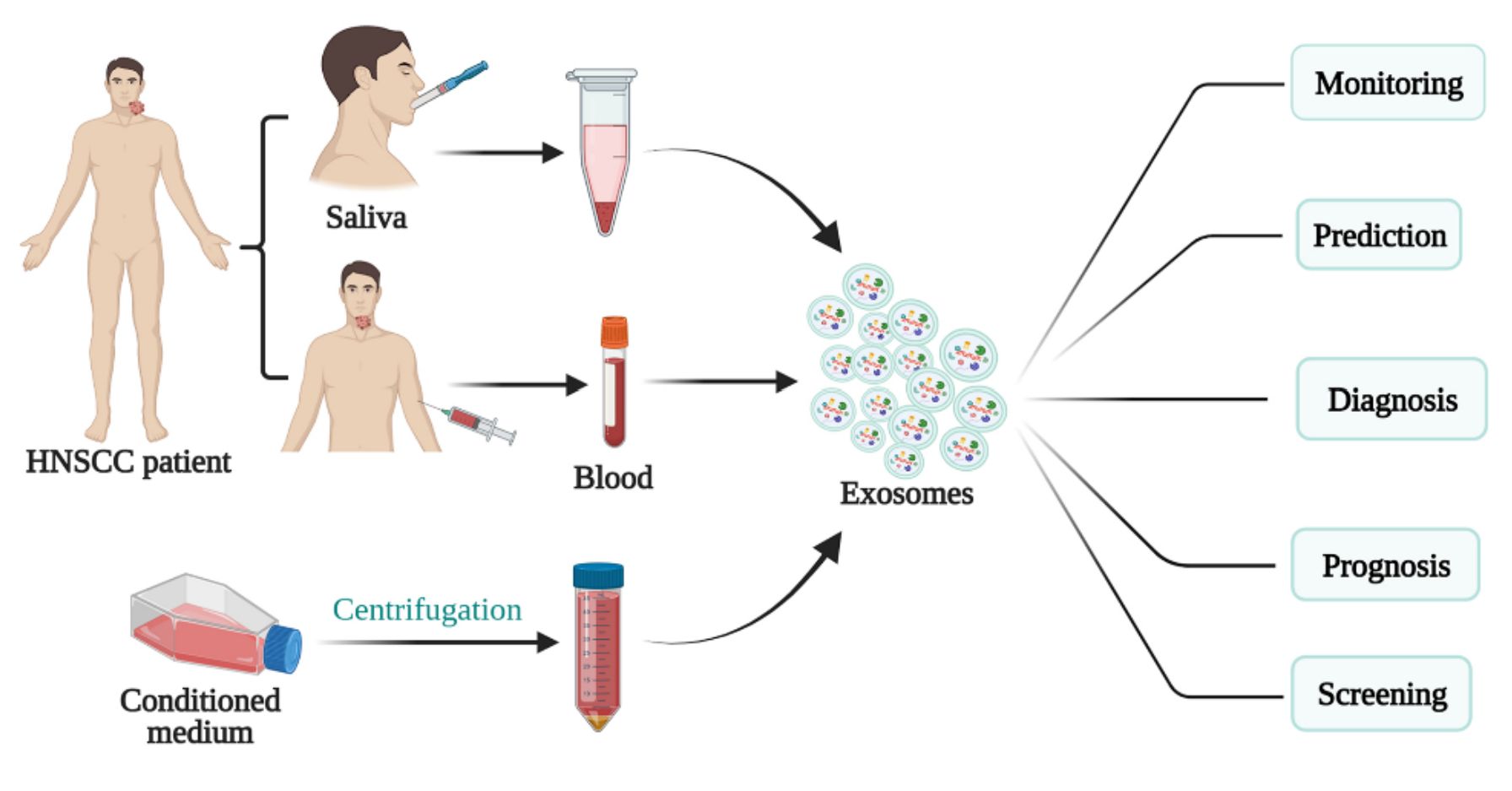 Figure 3. Schematic of exosomes for HNSCC diagnosis, prognosis, and treatment. (Teng Y, et al., 2021)
Figure 3. Schematic of exosomes for HNSCC diagnosis, prognosis, and treatment. (Teng Y, et al., 2021)
Exosomes as Therapy Targets for HNC
Given the important role in cancer progression, exosomes have emerged as a potential therapeutic target for HNC. Exosome-based strategies have focused on preventing exosome release and blocking communication between HNC cells and receptor cells. For example, GW4869 has been widely used to inhibit exosomes, a symmetrical dihydroimidazolo-amide compound that interferes with the lipid composition necessary for exosome shedding. Inhibition of ABCA3, an intracellular protein involved in lipid transport, impairs exosome release. However, exosome-targeted cancer therapies remain challenging for HNC.
Exosomes as Drug Carriers for the Therapy of HNC
Exosomes are considered promising carriers for antitumor therapy due to the high biocompatibility, low immunogenicity, and ability to interact with target cells. Currently, exosomes carrying chemotherapeutic drugs are increasingly being tested in clinical trials as anticancer drug delivery systems, such as exosomes delivering adriamycin (DOX), curcumin, and paclitaxel (PTX), which have demonstrated promising performance in terms of improving therapeutic efficacy and minimizing side effects. Research has shown that exosomes from normal tongue epithelial cells can exogenously deliver miR-200c to PTX-resistant squamous cell carcinoma of the tongue (TSCC) cells, increasing sensitivity to PTX treatment.
As a leader in the field of exosome research, Creative Biostructure has cutting-edge exosome technology and high-quality exosome products to ensure personalized solutions for our clients. We are committed to helping researchers advance to make significant progress in the fight against challenging HNC. Whether you are exploring molecular mechanisms or developing innovative therapeutic strategies, we are a trusted exosome research partner. Please feel free to contact us for more information.
References
- Xiao C, et al. Exosomes in Head and Neck Squamous Cell Carcinoma. Front Oncol. 2019. 9: 894.
- Cao J, et al. Exosomes in head and neck cancer: Roles, mechanisms and applications. Cancer Lett. 2020. 494: 7-16.
- Teng Y, et al.The Hidden Link of Exosomes to Head and Neck Cancer. Cancers (Basel). 2021. 13(22): 5802.
- Li T, et al. Exosomes: Potential Biomarkers and Functions in Head and Neck Squamous Cell Carcinoma. Front Mol Biosci. 2022. 9: 881794.
