Structural Research of Histidine Kinase Receptors
Histidine kinase receptors play a pivotal role in bacterial and archaeal signaling, enabling these microorganisms to perceive and respond to changes in their environment. Understanding the structural intricacies of these receptors is crucial for deciphering the underlying signaling mechanisms and harnessing their potential in various biotechnological applications.
Histidine kinases and chemoreceptors serve as ubiquitous environmental sensors in bacteria and archaea, playing critical roles in their survival and pathogenicity. These membrane-embedded proteins transmit signals from their sensor domains to the catalytic kinase domains over considerable distances, regulating the activity of the corresponding response regulator proteins in two-component signaling systems (TCSs).
Over the years, extensive research has been devoted to elucidating the structural dynamics of histidine kinase receptors. One fascinating area of investigation revolves around the proposed mechanisms of transmembrane signal transduction in TCS receptors. The swinging piston, helical rotation, and diagonal scissoring models have been put forward as possible mechanisms, yet a consensus on their universality remains elusive. Through rigorous crystallographic analysis of the sensor-TM-HAMP fragment of the R50S mutant, researchers made a remarkable discovery. Despite its inability to bind nitrate, the mutant NarQ exhibited a ligand-bound-like conformation. This unexpected finding sheds light on the diverse conformational dynamics of histidine kinase receptors and emphasizes the importance of thorough structural investigations. Comprehensive analysis revealed that the binding of nitrate to NarQ induces helical rotation and diagonal scissoring of the α-helices at the core of the sensor domain. These conformational changes are further accompanied by a subtle piston-like motion, which is amplified by a switch in the secondary structure of the linker between the sensor and TM domains. The interplay of these mechanisms demonstrates the versatility and complexity of histidine kinase receptors' signal transduction processes.
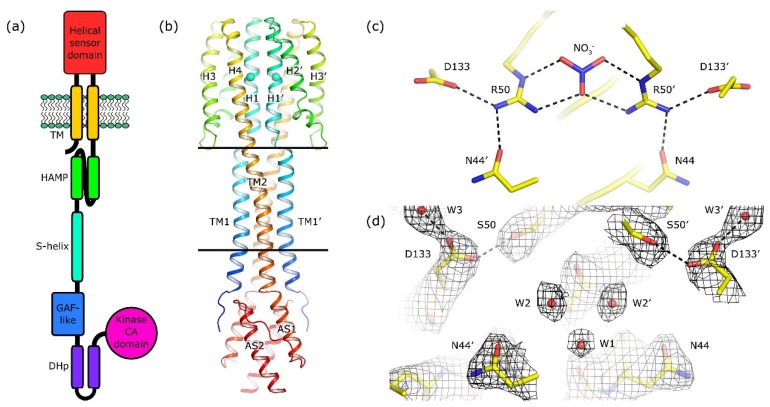 Figure 1. Architecture of NarQ and structure of the R50S mutant. (Gushchin I, et al., 2020)
Figure 1. Architecture of NarQ and structure of the R50S mutant. (Gushchin I, et al., 2020)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| ArcB (1-115) Aerobic Respiration Control sensor membrane domain (cell-free expression) | Escherichia coli K-12 | Solution NMR | / | 2KSD |
| QseC (1-185) Sensor protein membrane domain (cell-free expression) | Escherichia coli K-12 | Solution NMR | / | 2KSE |
| KdpD (397-502) Sensor protein membrane domain (cell-free expression) | Escherichia coli K-12 | Solution NMR | / | 2KSF |
| NarQ nitrate/nitrite sensor histidine kinase in symmetric holo state (expressed in Escherichia coli) | Escherichia coli | X-ray diffraction | 1.94 Å | 5IJI |
| NarQ histidine kinase proteolytic fragment (expressed in Escherichia coli) | Escherichia coli | X-ray diffraction | 2.30 Å | 6XYN |
| histidine kinase NarQ (R50S variant) fragment (expressed in Escherichia coli) | Escherichia coli | X-ray diffraction | 2.40 Å | 6YUE |
Table 1. Structural research of histidine kinase receptors.
Creative Biostructure extends an invitation to researchers and pharmaceutical companies for collaboration and utilization of our outstanding structural analysis services. Drawing from extensive industry experience, we have pioneered innovative methodologies and state-of-the-art techniques to conduct structural analysis of these receptors. Our team of expert scientists employs advanced technologies, such as cryo-electron microscopy (cryo-EM), X-ray crystallography and NMR spectroscopy, to attain high-resolution structural data, offering invaluable insights into the architecture and functionality of histidine kinase receptors. Contact us to explore how our cutting-edge capabilities can empower your research and propel you closer to accomplishing your scientific objectives.
References
- Gushchin I, et al. Mechanism of transmembrane signaling by sensor histidine kinases. Science. 2017, 356(6342): eaah6345.
- Gushchin I, et al. Sensor histidine kinase NarQ activates via helical rotation, diagonal scissoring, and eventually piston-like shifts. International Journal of Molecular Sciences. 2020, 21(9): 3110.