MemPro™ 5-Lipoxygenase–Activating Protein
Creative Biostructure provides custom MemPro™ gene-to-structure services for 5-Lipoxygenase–Activating Protein.
5-Lipoxygenase–Activating Protein, abbreviated as FLAP, is an internal nuclear membrane protein similar to leukotrienes C4 (LTC4) synthase. It belongs to MAPEG (membrane-associated proteins in eicosanoid-and-glutathione metabolism) superfamily which typically have glutathione transferase activity. Comparing the structure of LTC4 synthase with bound glutathione, FLAP in complex with MK-591 shows that these MAPEGs are structural homologues, although their primary sequences share quite limited identity and they play distinct biological roles in leukotriene synthesis. In contrast to the other five human MAPEGs, FLAP has no known enzymatic activity and doesn’t bind to any ligands as we know. Additionally, FLAP knockdown also shows limited negative effects in the presence of exogenous arachidonic acid, but led to prominent reductions in 5-LO product formation from endogenous substrate. Similar effects were observed in human as well. In addition, FLAP knockdown decreased in vivo synthesis of LTC4 from LTA4, suggesting a role for the activity of LTC4 synthase. Besides these results, the only known roles of FLAP are to selectively transfer arachidonic acid (AA) to 5-lipoxygenase (5-LO) and dehydration to leukotriene A4 (LTA4) and enhance the sequential oxygenation of AA to 5(S)-hydroperoxyeicosatetraenoic acid (5-HpETE).
FLAP was identified through a specific binding affinity with MK-886, a leukotriene biosynthesis inhibitor that blocks the binding of AA to FLAP in a concentration-dependent manner and that also inhibits the association of FLAP with 5-LO at a relative higher concentration. The structure-function relationship of binding sites on FLAP for indole and quinoline-based inhibitors led to the design of hybrid quinoline–indole compounds, such as by MK-591. FLAP contains four transmembrane helices that are connected by two elongated cytosolic loops and one short lumenal loop. Analysis of sequence similarity indicates that the secondary structure of FLAP is probably shared among other MAPEGs, but FLAP does not display significant structural homology with other protein containing transmembrane helical bundles. Compared with other human MAPEGs, the conserved FLAP is not observed and there is no electron density in glutathione binding region.
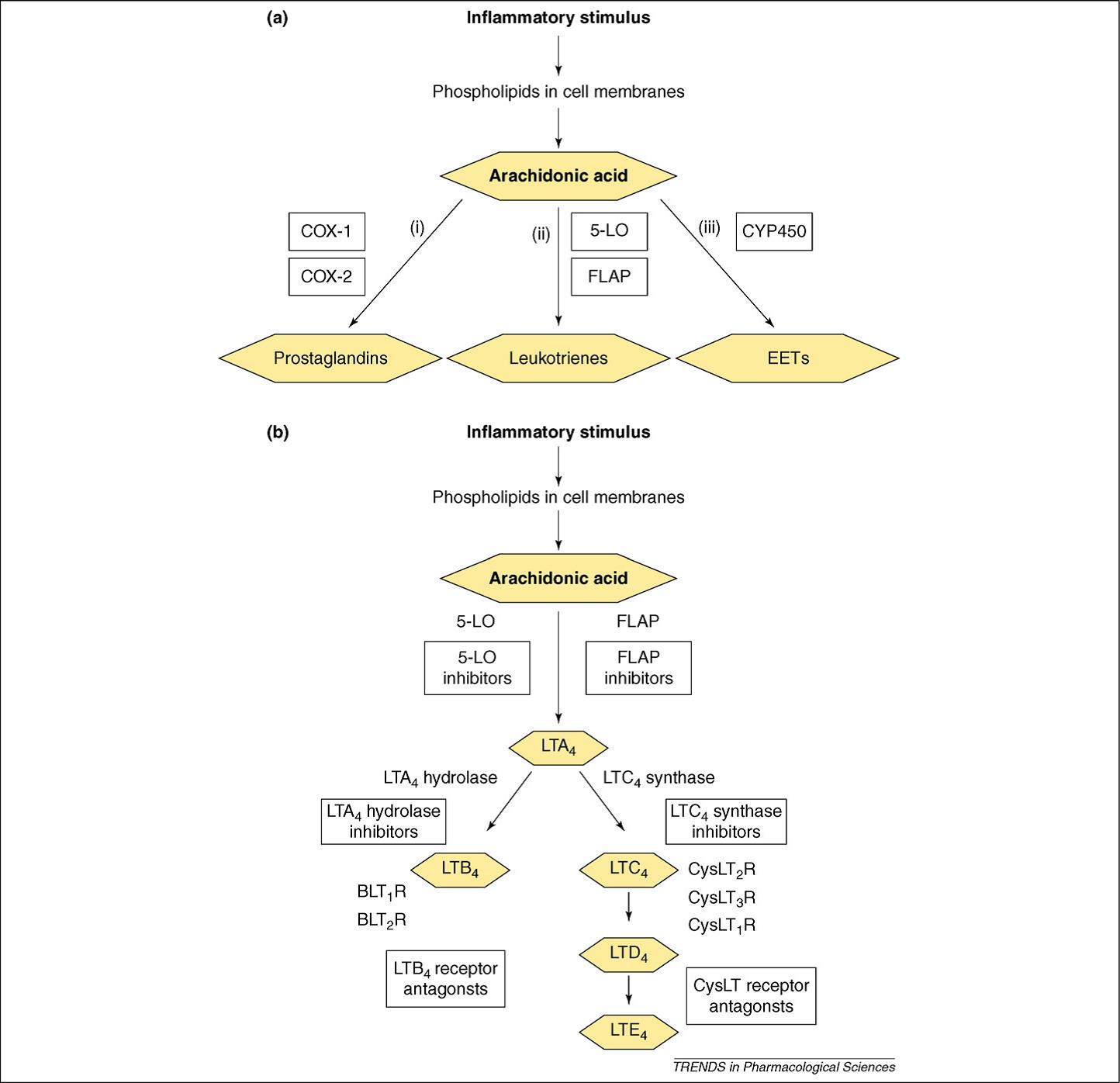
Figure 1. Profile of leukotriene pathway
Leukotrienes have pathophysiological roles in respiratory diseases, allergic diseases and cardiovascular diseases (CVDs). It was recognized that FLAP inhibitors weakly inhibited LTC4 synthase. Additionally, FLAP inhibitors have shown significant beneficial effects in both acute and chronic CVD animal models and for now three FLAP inhibitors for which phase I and phase II clinical data in asthma is available: the indole MK-886, the quinoline BAY X1005 and the quinoline– indole MK-591. All of three had good clinical safety profiles with no hepatotoxicity, and all showed promising activity versus early and late phase lung volume decreases after allergen challenge. In addition to their therapeutic benefit in respiratory disease, FLAP inhibitors have a great potential in the prevention and treatment of cardiovascular disease.
References:
Evans J F, Ferguson A D, Mosley R T, et al. What's all the FLAP about?: 5-lipoxygenase-activating protein inhibitors for inflammatory diseases[J]. Trends in pharmacological sciences, 2008, 29(2): 72-78.
Ferguson A D, McKeever B M, Xu S, et al. Crystal structure of inhibitor-bound human 5-lipoxygenase-activating protein[J]. Science, 2007, 317(5837): 510-512.
