MemPro™ HER4/ErbB4
Creative Biostructure provides custom MemPro™ gene-to-structure services for HER4/ErbB4.
HER4/ErbB4 is a ubiquitously expressed member of the epidermal growth factor (EGF/ErbB) family of receptor tyrosine kinases that is crucial for normal development of the heart, nervous system, and mammary gland. Along with other family EGF family member: EGFR, ErbB2 and ErbB3, any abnormal expression of these receptors are associated with various human cancers. Loss of ErbB4 function in particular results in defects in the heart, nervous system, and mammary gland in mice.
ErbBs mediate their biological effects through ligand-dependent elevation of their tyrosine kinase activities. ErbBs consist of an extracellular ligand-binding region, a single membrane spanning region, a cytoplasmic kinase domain, and a 220–350 amino acid C-terminal ‘‘tail’’ that becomes tyrosine phosphorylated following activation and mediates interactions between ErbBs and downstream effectors. Ligand binding to the extracellular region results in formation of specific ErbB homo- and heterodimers, activation of the cytoplasmic kinase, and initiation of intracellular signaling cascades. Both ErbB1 and ErbB4 behave in an archetypal manner and homodimerize and signal in response to ligand binding. ErbB2, however, lacks a known ligand and fails to homodimerize in normal circumstances.
ERBB4 can undergo intramembrane proteolysis, releasing a soluble intracellular domain (ICD) that modulates transcription in the nucleus. ERBB4 activated the transcriptional coactivator YAP, which promotes organ and tissue growth and is inhibited by the Hippo tumor-suppressor pathway. Heregulin, produced by normal epidermal cells, fails to induce phosphorylation of ErbB2 without ErbB4. They show diverse functions in the development of nervous system and play essential roles in vertebrate embryogenesis including: cardiac development, Schwann cell and oligodendrocyte differentiation.
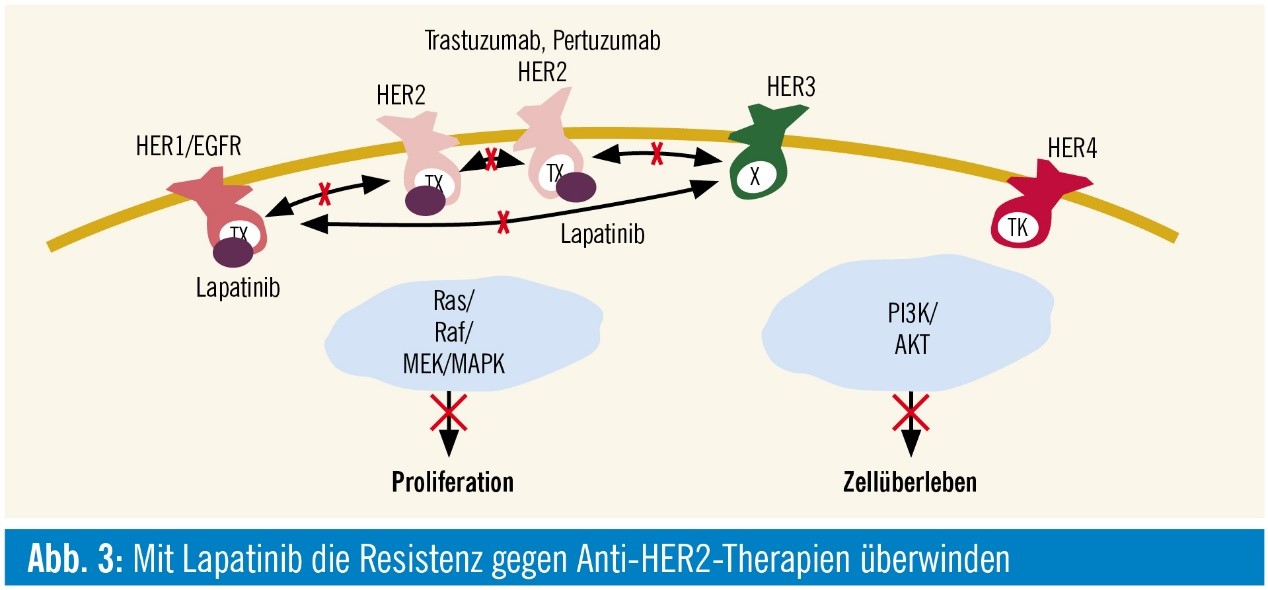 Figure.1 Lapatinib interaction with HER family
Figure.1 Lapatinib interaction with HER family
Crystallographic studies of the extracellular regions of ErbB1, ErbB3, and ErbB4 show them to be made up of four discrete subdomains that adopt an autoinhibited conformation in the absence of ligand. Ligand binding promotes a domain rearrangement that exposes previously obscured surfaces and allows them to mediate dimerization. In contrast, crystal structures of the ErbB2 extracellular region show it to adopt a fixed, active-like structure in which the canonical ligand-binding surfaces are involved in an interdomain interaction. These unique interactions rationalize the absence of an ErbB2 ligand and the role of ErbB2 as a universal ErbB heterodimerization partner.
Lapatinib is a dual tyrosine kinase inhibitor which interrupt the all the ErbB pathways. Although lapatinib appears to bind preferentially to ErbB2 and EGF, it shares the same binding sites with ErbB4. And all residues that come into van der Waals contact with lapatinib are conserved between EGFR and ErbB4 (and ErbB2). In 2010, FDA approved to lapatinib tablets for use in combination with letrozole for the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer that overexpresses the EGF receptor and for whom hormonal therapy is indicated.
Reference:
Haskins J W, Nguyen D X, Stern D F. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway targetgenes and promote cell migration[J]. Science signaling, 2014, 7(355): ra116.
Qiu C, Tarrant M K, Choi S H, et al. Mechanism of activation and inhibition of the HER4/ErbB4 kinase[J]. Structure, 2008, 16(3): 460-467.