Structural Research of G Protein-coupled Receptors (GPCRs) Class C
G Protein-coupled Receptors (GPCRs) are a superfamily of cell surface receptors that play critical roles in cell signaling and are essential elements involved in cell-to-cell communication. They represent major targets for therapeutic drugs due to their involvement in numerous physiological processes. GPCRs can be classified into several classes based on sequence homology, with class C GPCRs being a distinct subset that presents unique challenges in structural studies.
Recent advancements in structural research have greatly enhanced our understanding of GPCRs and their activation mechanisms. Notably, class C GPCRs, like the GABAB receptor, are obligatory dimers composed of two identical or similar subunits. The molecular basis of their asymmetric mode of action, where they activate only one G protein at a time, has remained a subject of curiosity and investigation.
Structural studies using techniques such as X-ray crystallography and cryo-electron microscopy (cryo-EM) have provided critical insights into the activation of GPCRs. In particular, the crystal structures of GPCRs in complex with G proteins have revealed the common mode of action involving interactions between the C-terminal extremity of the Gα subunit and a cavity on the intracellular side of the receptor. This interaction results from the movement of specific transmembrane helices relative to each other. However, for class C GPCRs, the activation mechanism is more complex. The cryo-EM structure of the GABAB receptor in complex with the G protein Gi1 has unveiled a unique mode of G-protein coupling. This study has demonstrated that small movements of transmembrane helices TM3 and TM5 cause changes in the intracellular loops (ICLs), leading to a binding site for the G protein on the GB2 subunit of GABAB.
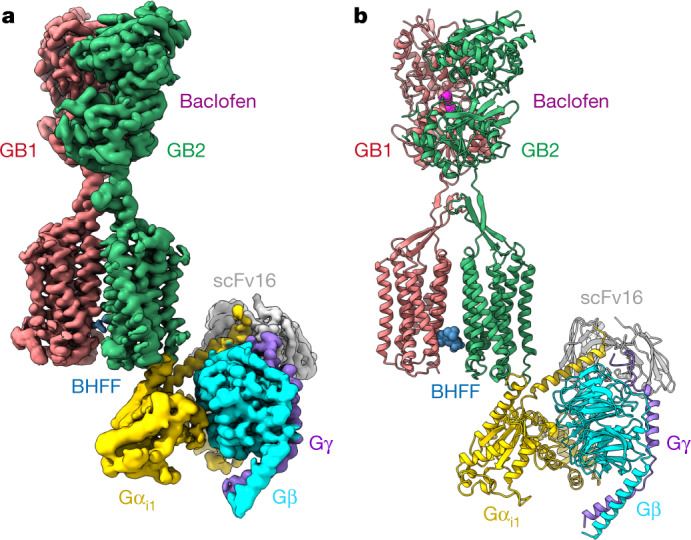 Figure 1. Cryo-EM structure of GABAB-Gi complex. (Shen C, et al., 2021)
Figure 1. Cryo-EM structure of GABAB-Gi complex. (Shen C, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Extracellular domain of human GABA(B) receptor in the apo form (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 2.35 Å | 4MQE |
| GABAB receptor peptide bound to KCTD16 T1 (expressed in Escherichia coli) | Homo sapiens | X-ray diffraction | 2.35 Å | 6OCP |
| Baclofen/BHFF-bound human GABA(B) receptor in active state (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.00 Å | 7C7Q |
| GABA(B) receptor in an inactive state (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.30 Å | 6WIV |
| GABAB1 homodimeric receptor in an inactive state (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.20 Å | 6W2Y |
| Human metabotropic GABA(B) receptor bound to agonist SKF97541 and positive allosteric modulator GS39783 (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.63 Å | 6UO8 |
| GABA(B) receptor bound to the positive allosteric modulator rac-BHFF (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 4.50 Å | 7CA3 |
| GABA(B) receptor-Gi protein complex (expressed in Trichoplusia ni) | Homo sapiens | Cryo-EM single particle analysis | 3.50 Å | 7EB2 |
| Human class C G protein-coupled metabotropic glutamate receptor 1 in complex with a negative allosteric modulator (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 2.80 Å | 4OR2 |
| Metabotropic Glutamate Receptor 1 (mGlu1), apo state (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.96 Å | 7DGD |
| Metabotropic Glutamate Receptor 2 (mGlu2) with bound Gi (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.50 Å | 7E9G |
| Full-Length mGlu2 in Inactive-State Bound to Antagonist LY341495 (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.65 Å | 7MTQ |
| Metabotropic Glutamate Receptor 2 (mGlu2) homodimer (expressed in mammal environmental sample) | Homo sapiens | Cryo-EM single particle analysis | 3.60 Å | 7EPA |
| Metabotropic Glutamate Receptor 3 (mGlu3) with bound LY341495 (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 4.17 Å | 7WI8 |
| Gi-bound metabotropic glutamate receptor mGlu4 (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 4.00 Å | 7E9H |
| Metabotropic Glutamate Receptor 5 (mGlu5) with bound negative allosteric modulator (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 2.60 Å | 4OO9 |
| Metabotropic Glutamate Receptor 5 (mGlu5) with bound compound 14 (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 3.10 Å | 5CGC |
| Metabotropic Glutamate Receptor 5 (mGlu5) apo form (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 4.00 Å | 6N52 |
| mGluR5 in complex with MMPEP (expressed in Spodoptera frugiperda) | Homo sapiens | X-ray diffraction | 2.22 Å | 6FFI |
| Metabotropic Glutamate Receptor 5 (mGlu5) with orthosteric antagonist, LY341495 (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 4.00 Å | 7FD9 |
| Metabotropic Glutamate Receptor 7 (mGlu7) inactive homodimer (expressed in mammal environmental sample) | Homo sapiens | Cryo-EM single particle analysis | 4.00 Å | 7EPC |
| Calcium-Sensing Receptor bound with calcium ions (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.80 Å | 7DTT |
| Calcium-Sensing Receptor in complex with NPS-2143 (expressed in HEK293 cells) | Gallus gallus | Cryo-EM single particle analysis | 3.20 Å | 7DD5 |
| Calcium-Sensing Receptor (CaSR) homodimer, active state-cinacalcet (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 2.80 Å | 7M3F |
| Calcium-Sensing Receptor (CaSR) homodimer in complex with NB2D11, inactive (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 6.00 Å | 7E6U |
| Positive allosteric modulator-bound active human calcium-sensing receptor (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 2.70 Å | 7SIL |
| Orphan GPR158 receptor (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.52 Å | 7EWL |
| Orphan GPR158 receptor (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.40 Å | 7SHE |
Table 1. Structural research of class C GPCRs.
At Creative Biostructure, we understand the significance of GPCRs as drug targets, particularly for the treatment of brain diseases and other medical conditions. Our extensive experience and dedication to cutting-edge research allow us to tackle complex biological questions and provide comprehensive structural insights.
With access to state-of-the-art equipment and a diverse range of structural biology techniques, including X-ray crystallography and cryo-electron microscopy (cryo-EM), we are well-equipped to handle even the most challenging structural analysis projects. Our team of experts ensures the highest quality data and interprets the results with precision, enabling our clients to make informed decisions in drug discovery and therapeutic development. Contact us to discover how our cutting-edge capabilities can empower your research and drive you closer to achieving your scientific goals.
References
- Patil D N, et al. Cryo-EM structure of human GPR158 receptor coupled to the RGS7-Gβ5 signaling complex. Science. 2022, 375(6576): 86-91.
- Shen C, et al. Structural basis of GABAB receptor–Gi protein coupling. Nature. 2021, 594(7864): 594-598.