Structural Research of Multi-Drug Efflux Transporters
Multi-drug efflux transporters are membrane proteins found in many bacteria and human cells. They cause multiple drug resistance (MDR) in bacteria and cancer cells. MRD is a serious problem in cancer chemotherapy and the treatment of bacterial infections.
Research Progress of Multi-drug Efflux Transporters in Bacteria
In Gram-negative bacteria, tripartite multidrug effector systems export biological metabolites and antimicrobial compounds, thereby promoting bacterial resistance. RND transporters are proton or drug efflux transporters. MexB, OprM, and MexA are synergistically involved in Pseudomonas aeruginosa resistance as RND. MexB is an asymmetric homologous trimer, with the three monomer conformations of the continuous state designated as Loose (or Access), Tight (or Binding), and Open (or Extrusion). The hydrophobic pockets created in the T monomer are pockets based on ACRB-drug complex binding.
Function and Structure of Multi-drug Efflux Transporter ABCG2
Adenosine triphosphate (ATP) binding cassette efflux transporter G2 (ABCG2) was originally discovered in multidrug-resistant breast cancer cell lines. ABCG2 is expressed in inflammation, infection, tissue injury, and in response to allogeneic and endogenic hormones. ABCG2 is a slit-like binding cavity, acting as the binding site for both substrates and inhibitors. The binding cavity houses flat, polycyclic, hydrophobic small molecules that are stacked between two protruding phenylalanine side chains.
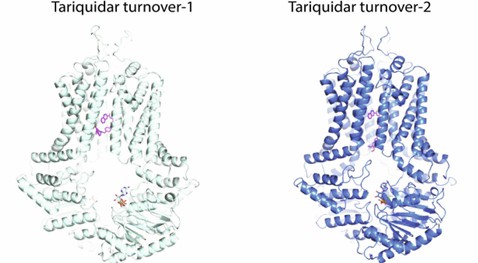 Figure 1. Models of tariquidar-bound ABCG2 in TO1 (Left) and TO2 (Right) states. (Rasouli A, et al., 2023)
Figure 1. Models of tariquidar-bound ABCG2 in TO1 (Left) and TO2 (Right) states. (Rasouli A, et al., 2023)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| ABCG2 turnover-1 state with tariquidar bound | Homo sapiens | Cryo-EM single particle analysis | 3.10 Å | 8BHT |
| ABCG2 turnover-2 state with tariquidar bound | Homo sapiens | Cryo-EM single particle analysis | 3.20 Å | 8BI0 |
| Apo-closed ABCG2 | Homo sapiens | Cryo-EM single particle analysis | 3.50 Å | 6VXF |
| Inhibitor-bound ABC transporter | Homo sapiens | Cryo-EM single particle analysis | 3.56 Å | 6FFC |
| ABCG2-MZ29-Fab complex with cholesterol and PE lipids docked | Homo sapiens | Cryo-EM single particle analysis | 3.56 Å | 6HIJ |
| Multidrug Exporter MexB | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 3.00 Å | 2V50 |
| Multidrug exporter AcrB reveals a proximal multisite drug-binding pocket | Escherichia coli K-12 | X-ray diffraction | 3.35 Å | 3AOA |
| Inhibition of bacterial multidrug exporters | Pseudomonas aeruginosa PAO1 | X-ray diffraction | 2.71 Å | 3W9I |
| Gdx-Clo of transporters in complex with octylguanidinium | Clostridiales bacterium oral taxon 876 str. F0540 | X-ray diffraction | 2.32 Å | 6WK9 |
| Multiple Binding Capacity of the AcrB Multidrug Efflux Pump | Escherichia coli | X-ray diffraction | 3.68 Å | 1OY6 |
| multidrug transporter reveals a functionally rotating mechanism | Escherichia coli | X-ray diffraction | 2.80 Å | 2DHH |
| Inhibition of bacterial multidrug exporters | Escherichia coli K-12 | X-ray diffraction | 3.05 Å | 3W9H |
Table 1. Structural research of multi-drug efflux transporters.
To study the structure of membrane proteins, cryo-electron microscopy (cryo-EM) and X-ray crystallography are commonly used. Structural analysis of multi-drug efflux transporters is beneficial to the study of the role of bacterial resistance. It also helps in the development of inhibitors (EPI).
Creative Biostructure has long been committed to the study of structural biology and membrane proteins. We have extensive experience in determining membrane protein structures using single particle analysis (SPA).
In addition to the structural determination of membrane proteins, we can accurately analyze biomolecules, including but not limited to nucleic acids, ribosomes, small proteins, protein complexes, protein-ligand complexes, and viruses. If you are interested in our services, please contact us and we will provide you with a professional and comprehensive solution.
References
- Nishino K, et al. Function and Inhibitory Mechanisms of Multidrug Efflux Pumps. Frontiers in Microbiology. 2021;12:737288.
- Rasouli A, et al. Differential dynamics and direct interaction of bound ligands with lipids in multidrug transporter ABCG2. Proc Natl Acad Sci U S A. 2023;120(1): e2213437120.
- Delmar JA, et al. Bacterial multidrug efflux transporters. Annu Rev Biophys. 2014;43:93-117.
- Kukal S, et al. Multidrug efflux transporter ABCG2: expression and regulation. Cell Mol Life Sci. 2021;78(21-22):6887-6939.