Structural Research of High-Density Lipoprotein (HDL) Receptors
High-Density Lipoprotein (HDL) receptors play a pivotal role in lipid metabolism, particularly in the context of cholesterol homeostasis and cardiovascular health. Understanding the structural basis of the interaction between HDL and its receptor, scavenger receptor BI (SR-BI), is of utmost importance in the pursuit of novel therapeutic approaches for combating cardiovascular diseases.
The interaction of HDL with its receptor, SR-BI, has been extensively investigated in recent years to decipher the molecular mechanisms governing cholesterol transport and cellular uptake. Researchers have employed cutting-edge techniques, such as nuclear magnetic resonance (NMR) spectroscopy, X-ray crystallography, and homology modeling, to gain insights into the structural aspects of HDL receptors.
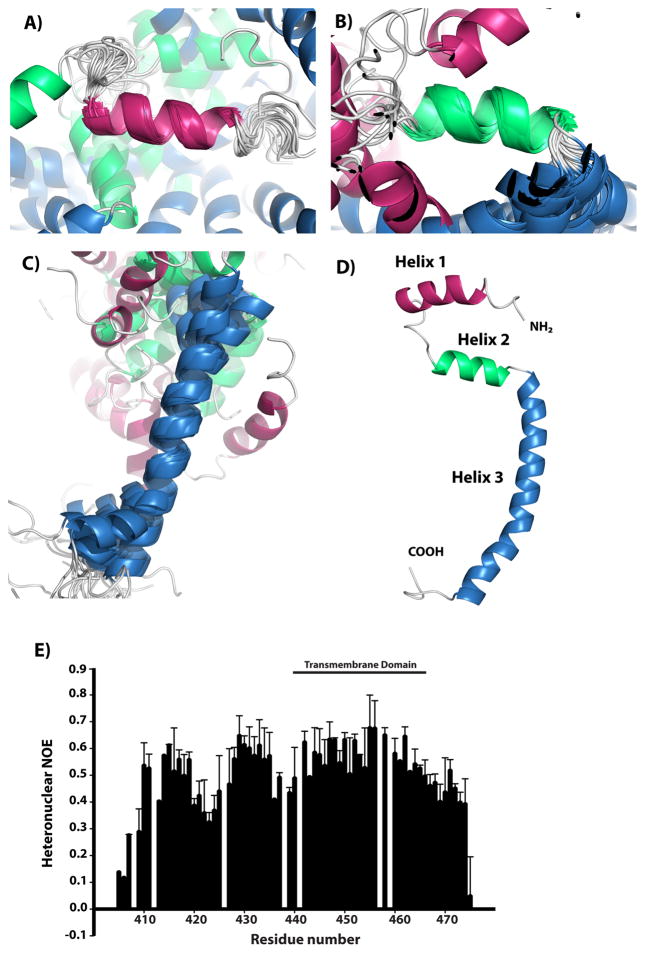 Figure 1. The solution NMR structure of SR-BI (405-475). (Chadwick A C, et al., 2017)
Figure 1. The solution NMR structure of SR-BI (405-475). (Chadwick A C, et al., 2017)
NMR Spectroscopy Reveals the C-Terminal Transmembrane Domain
One groundbreaking advancement in structural research involves the use of NMR spectroscopy to elucidate the high-resolution structure of the C-terminal transmembrane domain of SR-BI. The C-terminal region harbors a crucial leucine zipper dimerization motif, which facilitates the formation of receptor dimers essential for HDL binding and cholesterol delivery. Mutations in this motif have been shown to impair the ability of SR-BI to bind HDL and mediate cholesterol transport, underscoring the importance of this structural domain.
Homology Modeling and Insights from Related Receptor Proteins
While a complete high-resolution structure of SR-BI remains elusive, homology modeling using related scavenger receptor family members, such as lysosome integral membrane protein type 2 (LIMP-2), has provided valuable information. By leveraging the known crystal structure of LIMP-2, researchers have improved their understanding of the extracellular domain of SR-BI. However, a comprehensive structural depiction of the transmembrane domains and cytoplasmic regions of SR-BI is still lacking, warranting further investigation.
Homo-Oligomerization and Hydrophobic Channels
Another intriguing aspect of HDL receptors is their ability to form homo-oligomers, a phenomenon observed in various cell types. These homo-oligomers are believed to create a non-aqueous "hydrophobic channel," which facilitates the transport of cholesterol between HDL and the plasma membrane. The transmembrane domains, particularly the C-terminal region, have been implicated in SR-BI homo-oligomerization. Fluorescence resonance energy transfer studies in live cells have demonstrated dimerization through interactions between the C-terminal transmembrane domains.
| Protein | Organism | Method | Resolution | PDB Entry ID |
| C-terminal transmembrane domain of scavenger receptor BI (SR-BI) (expressed in Escherichia coli) | Mus musculus | Solution NMR | / | 5KTF |
Table 1. Structural research of high-density lipoprotein (HDL) receptors.
Creative Biostructure is a renowned entity in the realm of structural biology, providing cutting-edge structural analysis services, such as NMR spectroscopy, X-ray crystallography, and homology modeling. Our proficient team of experts, with extensive industry experience, excels in unraveling intricate structural intricacies of biomolecules. Contact us today to explore the potential of our advanced capabilities, enabling your research and propelling you towards accomplishing your scientific aspirations.
Reference
- Chadwick A C, et al. NMR structure of the C-terminal transmembrane domain of the HDL receptor, SR-BI, and a functionally relevant leucine zipper motif. Structure. 2017, 25(3): 446-457.
