Structural Research of Leviviridae
The Leviviridae are a family of positive-stranded RNA viruses that infect prokaryotes, consisting of two genera, Allolevivirus and Levivirus. Of these, the RNA-binding capsid protein (CP) of the Levivirus MS2 phage is a tool for many basic science applications and a model for RNA virus assembly. It is evident from recent research that Leviviridae phages are commonly used as indicators of the presence of viruses in wastewater and surface water, as these species favor specific hosts. In 2020, the family was renamed from Leviviridae to Fiersviridae.
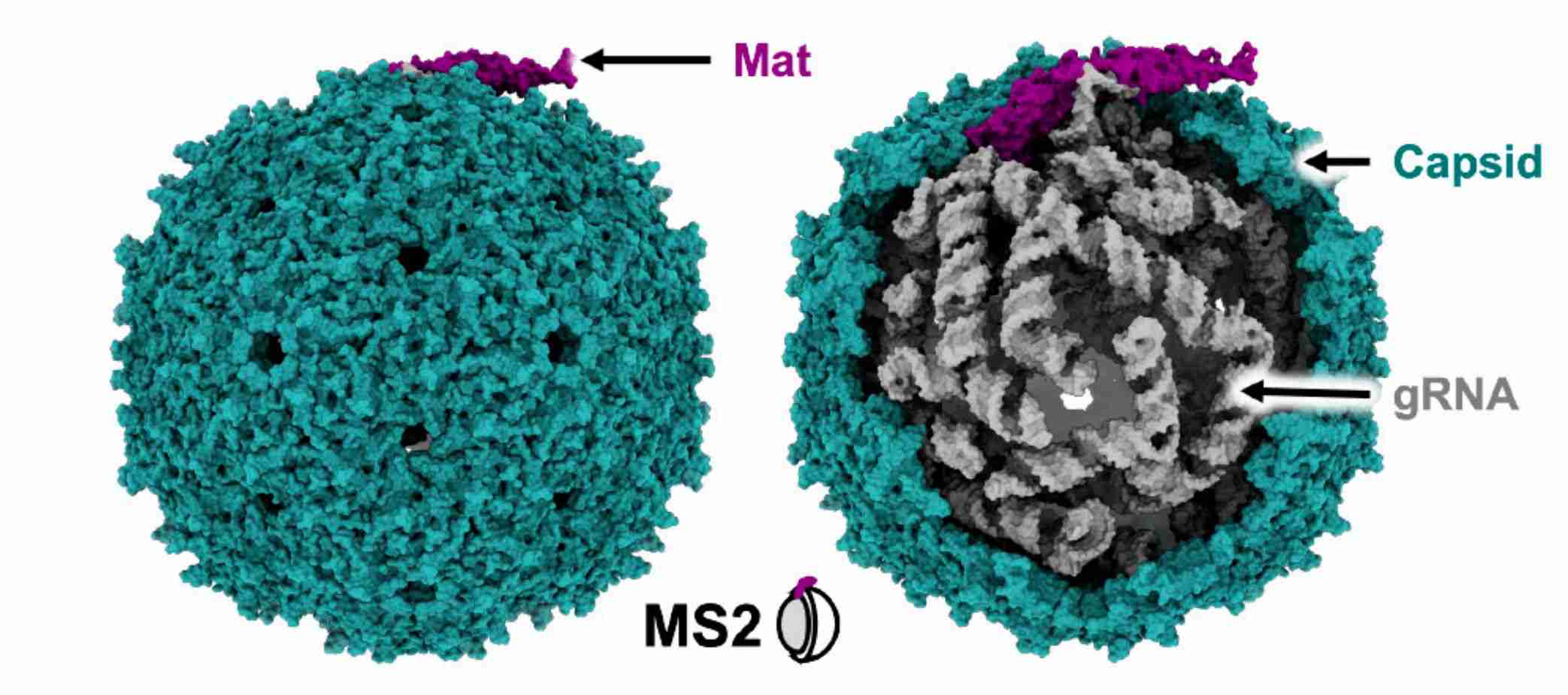 Figure 1. Atomic model of Levivirus MS2. (Thongchol J, et al, 2023)
Figure 1. Atomic model of Levivirus MS2. (Thongchol J, et al, 2023)
Structure and Composition of Leviviridae Virion
Leviviridae members are shown as unenveloped, spherical viral particles, approximately 26 nm in diameter, with T=3 icosahedral symmetry. Members of the family are small viruses with linear, positive-sense, single-stranded RNA genomes encoding four essential proteins, including the maturation protein (Mat), the CP, the β-subunit of the replicase (Rep), and a single cleavage protein (Lys). The Mat plays an essential role in determining phage maturation. Mat and CP are responsible for the structural integrity of the phage, and Rep replicates the viral genome within the host cell. The newly formed viral progeny is released into the environment via Lys, which is required for host cell lysis.
Research Progress on the Structure of Leviviridae
In the early years, researchers used X-ray crystallography and cryo-electron microscopy (cryo-EM) to resolve the capsid structures of known Leviviridae phages. One of the most widely researched phages was the Escherichia coli phage MS2. The cryo-EM structures of MS2 provided insights into the composition of the virion particles of mature Leviviridae phages. The structure shows that the MS2 virus particle comprises a capsid encapsulating a single-stranded gRNA and a single Mat. The capsid has a nearly icosahedral shape with 89 CP dimers, and the Mat breaks the T = 3 icosahedral symmetries of one of the 2-fold axes.
As a leader in viral structural biology, Creative Biostructure offers comprehensive viral structure analysis services and cutting-edge virus-like particles (VLPs) products. Our viral structure analysis services provide detailed insights into the three-dimensional arrangement of viral proteins, revealing the complex structure of viruses. This information is invaluable for understanding viral replication, host interactions, and developing antiviral strategies.
Our products are widely used in many areas of virology and related disciplines. In vaccine development, VLPs provide an excellent platform for antigen presentation, enabling research on immune responses and the design of effective vaccines. They also play an essential role in drug discovery by facilitating the identification of potential therapeutic targets and the screening of antiviral compounds.
| Cat No. | Product Name | Virus Name | Source | Composition |
| CBS-V543 | Bacteriophage MS2 VLP (coat protein Proteins) | Bacteriophage MS2 | E. coli recombinant | coat protein |
| CBS-V544 | Bacteriophage MS2 VLP (coat protein Proteins) | Bacteriophage MS2 | Yeast recombinant | coat protein |
| CBS-V546 | Bacteriophage Qβ VLP (coat protein Proteins) | Bacteriophage Qβ | E. coli recombinant | coat protein |
| Explore All Leviviridae Virus-like Particle Products | ||||
Based on our advanced electron microscopy (EM) platform, Creative Biostructure can support researchers, pharmaceutical companies, and diagnostic professionals to deepen the understanding of viral structure and action mechanisms and contribute to developing effective antiviral interventions. Please feel free to contact us for a detailed quote.
Reference
- Thongchol J, et al. Recent Advances in Structural Studies of Single-Stranded RNA Bacteriophages. Viruses. 2023. 15(10): 1985.