Structural Research of Arteriviridae
The members of Arteriviridae family are small, enveloped animal viruses, whose sense RNA genome is surrounded by an icosahedral capsid. The family includes equine arteritis virus (EAV; prototype), porcine reproductive and respiratory syndrome virus genotypes I and II (PRRSV-I and PRRSV-II), mouse lactate dehydrogenase elevated virus (LDV), and simian hemorrhagic fever virus (SHFV). Three of these viruses were first discovered and characterized in the 1950s and 1960s, and PRRSV was first isolated in Europe and North America in the early 1990s. The viruses of this family are highly species-specific but share many biological and molecular properties, including virion morphology, a unique set of structural proteins, genomic organization, replication strategies, and the ability to establish long-term or true persistent infections in their natural hosts. However, the epidemiology and pathogenesis of infections caused by each virus are different, and the diseases caused are also different.
Arterivirus structure
Arteriviruses are polymorphic, round, or egg-shaped particles, about 50-65 nm in diameter, and have a relatively smooth, mostly uncharacterized surface. The nucleocapsid structure of Arterivirus consists of an RNA genome with a length of 12.7-15.7 kb and nucleocapsid proteins (N protein). It has been previously thought that the nucleocapsid structure is of equal length, but the low-temperature electron tomography study of PRRSV indicates that the core structure of PRRSV is quite polymorphous and unorganized (an average of 39 nm in diameter, separated from the envelope by a 2-3 nm gap), which is incompatible with the icosahedral core, but similar to the nucleocapsid structure of the coronavirus, or more loose filamentous core structure. Based on studies of EAV and PRRSV, it is now speculated that the lipid bilayer envelope surrounding the nucleocapsid contains seven kinds of envelope proteins, which is a very large number compared to other sense RNA viruses. Two major envelope proteins and five minor envelope proteins are distinguished in the envelope proteins of Arterivirus. By knocking out the expression of each structural protein separately, it is determined that all major and minor structural proteins are necessary for the generation of infectious offspring (perhaps except for ORF5a protein). Two major envelope proteins, namely, GP5 and the non-glycosylated triple-spanning membrane protein M, form disulfide-linked heterodimers. The minor structural proteins, glycoproteins GP2, GP3, and GP4 form disulfide-linked heterotrimers GP2-GP3-GP4 in virions and further highlight their importance. In addition, GP2-GP4 dimer has also been identified in equine arteritis virus (EAV). In conclusion, the basic protein scaffold of Arterivirus consists of three major structural peptides, protein N, protein M, and GP5.
Porcine reproductive and respiratory syndrome virus
PRRSV is the largest of the four Arteriviruses, and porcine reproductive and respiratory syndrome (PRRS) caused by this virus is currently the most important infectious disease affecting the global swine industry. The PRRSV genome is approximately 15 kb in length and has a cap at the 5' end and a Poly (A) tail at the 3' end, including nine open reading frames (ORFs) that overlap end to end. ORFla and ORFlb encode non-structural proteins and replicases; ORF2a, ORF3, ORF4, and ORF5 encode the membrane-associated glycosylated structural proteins GP2a, GP3, GP4, and GP5; ORF2b and ORF6 encode the non-glycosylated membrane proteins E and M; ORF7 encodes the N protein. The major protein components of the PRRSV envelope are GP5 and protein M, which are dimeric forms in PRRSV-infected cells and are essential for the formation of virus-like particles. When either of them lacks, even a PRRSV strain with strong infection ability cannot form virus-like particles, and the absence of other minor envelope proteins will not affect virus replication. In recent years, research on PRRS virus-like particle (VLP)-based vaccine has become a hot topic, and some achievements have been made.
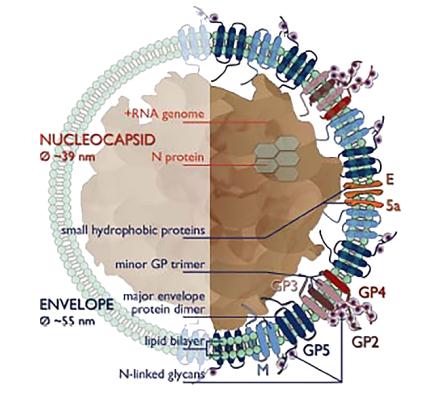 Figure 1. Arterivirus structure.
Figure 1. Arterivirus structure.
Highly purified VLPs can be utilized to study the structural properties of natural viruses, and they are a safe option for the research and development of vaccines since they can trigger powerful immune responses and do not contain viral genetic material. Creative Biostructure provides VLP products that are ready for immediate worldwide delivery. In addition, using our cryo-electron microscopy service platform, the ultrastructural information of viral particles can be obtained to provide a reference for the design and development of vaccines and antiviral drugs.
References
- Spilman M S, et al. Cryo-electron tomography of porcine reproductive and respiratory syndrome virus: organization of the nucleocapsid. Journal of General Virology. 2009. 90(3): 527-535.
- Binjawadagi B, et al. Development of a porcine reproductive and respiratory syndrome virus-like-particle-based vaccine and evaluation of its immunogenicity in pigs. Archives of Virology. 2016. 161(6): 1579-1589.