Cryo-electron tomography (cryo-ET) is a groundbreaking imaging technique in structural biology that allows for the 3D visualization of biological samples. By capturing a series of two-dimensional (2D) projection images across a tilt range (typically −60° to +60°), cryo-ET reconstructs a three-dimensional (3D) model of biological structures at nanometer resolution. This technique is particularly useful for studying complex, pleomorphic objects like the interior of cells, offering insights into their macromolecular architecture in near-native conditions. However, cryo-ET data processing comes with its own set of challenges. In this article, we'll explore the latest advances and best practices in cryo-ET data processing, focusing on overcoming low signal-to-noise ratios (SNR) and leveraging artificial intelligence (AI) to enhance resolution.
Overview of Cryo-ET and Its Core Benefits
Cryo-ET uses rapid freezing techniques, such as liquid ethane freezing, to preserve biological samples in vitreous ice, maintaining their native conformation. Paired with cryo-focused ion beam (Cryo-FIB) thinning, this approach allows lamella preparation from thick cellular or tissue regions, with controlled thickness (200-300 nm) to optimize electron penetration. The main advantages of cryo-ET include:
- In situ Analysis: Cryo-ET allows for the direct observation of molecular assemblies within intracellular membrane compartments (e.g., nuclear pore complexes) and non-membrane compartments (e.g., stress granules).
- Dynamic Observation: This technique is ideal for studying the formation of biomolecular condensates through liquid-liquid phase separation (LLPS), capturing dynamic molecular processes.
- High-Resolution Reconstruction: With the integration of subtomogram averaging (STA), cryo-ET can achieve sub-nanometer resolution (<1 nm), providing detailed structural insights.
Comparison to Cryo-EM SPA Data Processing
While cryo-ET shares similarities with single-particle cryo-EM (SPA) in terms of data collection and image reconstruction, there are key differences:
- Sample Homogeneity: SPA relies on obtaining a homogeneous sample with many particles in different orientations, whereas cryo-ET can accommodate heterogeneous samples and does not require the sample to be highly purified.
- Data Acquisition: Cryo-ET collects tilt-series of 2D images, providing 3D information, while SPA typically uses 2D projections of individual particles. This makes cryo-ET more suited for studying large, flexible structures in their native environments.
- Resolution: SPA generally achieves higher resolution due to the ability to average many identical particles, while cryo-ET is more challenging to process due to the lower SNR in individual images. However, recent advancements in cryo-ET data processing have closed this gap, enabling near-atomic resolution structures.
Select Service
The Whole Workflow of Cryo-ET Data Processing
Cryo-ET data processing involves a complex multi-step workflow to extract high-resolution structural information from biological samples. Each step is crucial for ensuring data accuracy and improving reconstruction quality. Below is a detailed breakdown of the entire workflow:
1. Sample Preparation and Data Acquisition
- Cryo-FIB Milling and Cryo-CLEM Localization: To prepare thin, electron-transparent samples, cryo-focused ion beam scanning electron microscopy (Cryo-FIB SEM) is used to accurately thin frozen cells. Correlated light and electron microscopy (Cryo-CLEM) then helps pinpoint the target area of interest for imaging.
- Tilt-Series Acquisition: Projection images are collected at tilt angles ranging from -60° to +60° in a transmission electron microscope (e.g., FEI Titan Krios), typically with a step size of 1-2°. The data volume generated per sample can range from 1-2 TB, making efficient data management essential.
2. Preprocessing and Alignment
- Motion Correction and Dose Compensation: During image acquisition, beam-induced motion can introduce blurring, affecting resolution. Warp and RELION are commonly used to apply motion correction and dose weighting, ensuring that low-dose areas contribute optimally to signal quality.
- Tilt-Series Alignment: Accurate geometric alignment is achieved using the Tiltalign module in IMOD, which matches fiducial markers (such as gold particles) across frames. When fiducials are unavailable, feature-based alignment techniques help improve accuracy.
3. Tomographic Reconstruction
- Back-Projection Algorithms: After alignment, 3D tomograms are generated using weighted back projection (WBP) or iterative reconstruction methods such as simultaneous iterative reconstruction technique (SIRT). These algorithms help convert 2D tilt images into 3D volumes while minimizing reconstruction artifacts.
- Noise Reduction and Image Enhancement: Since cryo-ET data suffers from low SNR, advanced noise reduction tools are applied. CryoCARE, a deep learning-based tool, enhances tomogram quality, while TomDecon performs deconvolution to improve structural clarity.
4. Subtomogram Averaging (STA)
- Particle Extraction and Classification: To improve resolution, sub-volumes containing the target macromolecule are extracted from the tomogram using PEET or RELION. These particles are then classified based on 3D similarity to identify structurally homogeneous subsets.
- Missing Wedge Correction: The limited tilt range introduces the missing wedge effect, leading to anisotropic resolution. This issue is addressed by applying symmetry constraints (such as helical symmetry) or by performing iterative refinement and averaging to compensate for missing data.
5. Resolution Optimization and Validation
- Local Structure Optimization: Molecular dynamics flexible fitting (MDFF) is used to refine structures by fitting known atomic models into the tomographic density map, enhancing detail accuracy.
- Resolution Assessment Using FSC: The Fourier Shell Correlation (FSC) method is applied to evaluate the resolution of the final 3D structure, with FSC = 0.143 as the standard threshold for determining reliable resolution.
Key Steps in Cryo-ET Data Processing
1. Motion Correction and Alignment
The first step in cryo-ET data processing involves correcting for any motion that may have occurred during the tilt-series acquisition. This includes correcting for both global and local movements, such as mechanical drift and beam-induced motion (BIM), which can distort the final images.
2. Contrast Transfer Function (CTF) Estimation
CTF estimation is crucial for correcting distortions introduced by the electron microscope. Accurate CTF correction ensures that the electron density maps generated from cryo-ET data are reliable and can be used for accurate structural analysis.
3. Tomogram Reconstruction and Subtomogram Averaging
Once the tilt-series has been aligned and CTF corrections are applied, the individual images are back-projected to create a 3D tomogram. STA involves extracting sub-volumes that contain the particle of interest, aligning them to a common reference, and averaging the results to improve the signal-to-noise ratio.
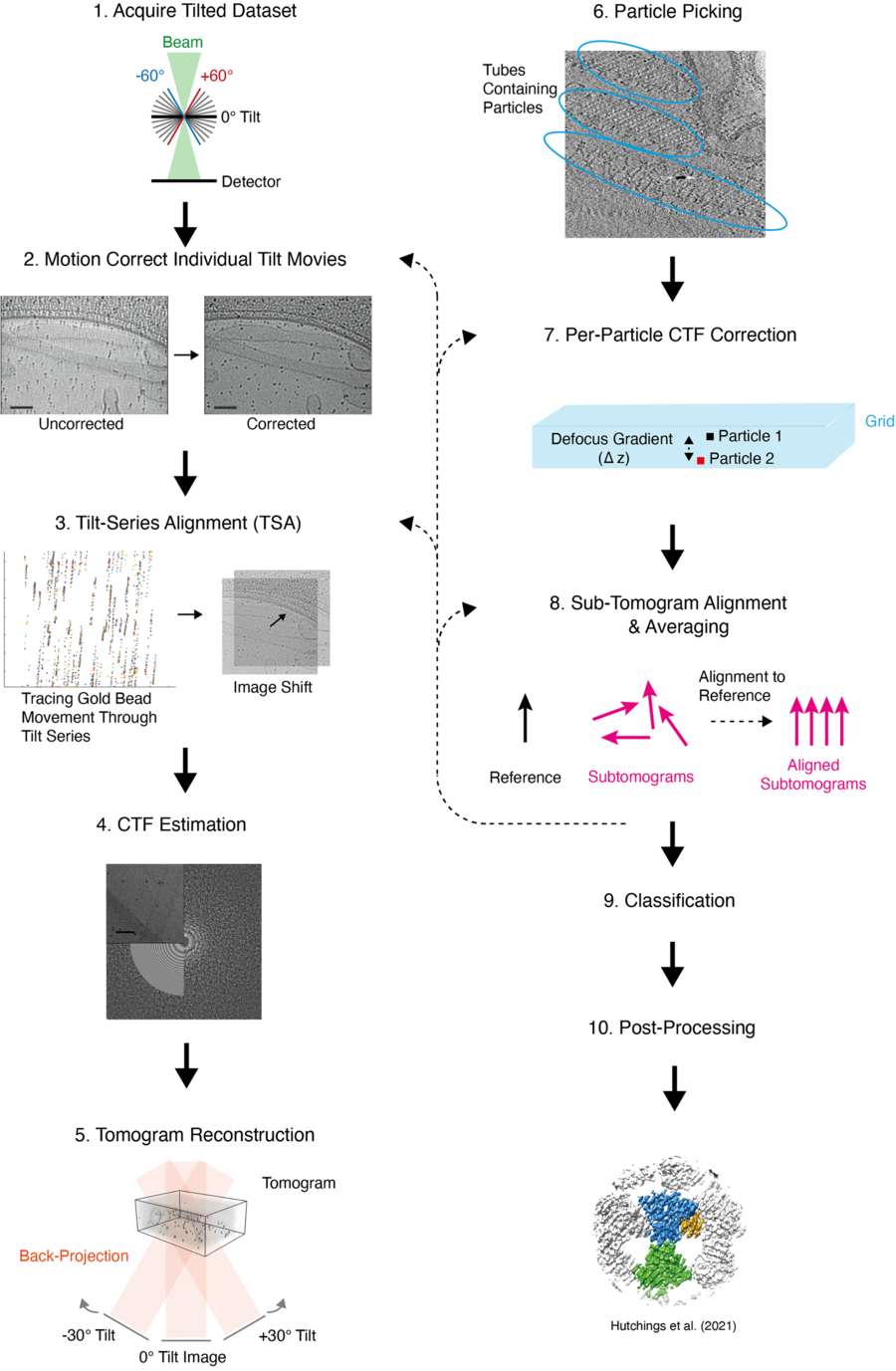 Figure 1. Overview of Cryo-ET with STA Data Processing Workflow. Detailed workflow for cryo-ET with STA, covering tilt-series collection, alignment, CTF correction, subtomogram averaging, and post-processing steps. (Pyle E, et al., 2021)
Figure 1. Overview of Cryo-ET with STA Data Processing Workflow. Detailed workflow for cryo-ET with STA, covering tilt-series collection, alignment, CTF correction, subtomogram averaging, and post-processing steps. (Pyle E, et al., 2021)
Challenges in Cryo-ET Data Processing
Cryo-ET data processing involves several challenges that must be addressed to extract reliable, high-resolution structures:
Low Signal-to-Noise Ratio and Missing Wedge Effects
The low SNR in cryo-ET is caused by the need to collect data from multiple tilt angles, resulting in a fractionation of electron dose over the tilt-series. This leads to noisy images, and combined with the "missing wedge" effect (due to the limited tilt range), it can cause anisotropic resolution in reconstructed tomograms. These issues complicate data interpretation and can reduce the accuracy of structural reconstructions.
High Computational Demands and Data Volume
Cryo-ET generates large datasets that require extensive computational resources for processing. The sheer volume of data generated by multiple tilt images and the large number of subtomograms needed for averaging can place a significant burden on computational systems, making data processing time-consuming and storage-intensive.
Recent Advances in Cryo-ET Data Processing
Despite these challenges, recent technological and methodological innovations have made significant strides in cryo-ET data processing.
Innovations in Sample Preparation and Data Collection
Recent advances in sample thinning, such as focused ion beam (FIB) milling, have significantly improved the quality of cryo-ET samples. FIB milling allows researchers to prepare thinner samples, enabling better electron penetration and reducing artifacts caused by thick samples. This, coupled with high-throughput parallel data collection strategies, has increased the efficiency of cryo-ET experiments, reducing the bottleneck at the data collection stage and shifting the focus to the more challenging data processing phase.
Development of Accessible Graphical User Interfaces (GUIs)
The rise of user-friendly software packages with intuitive graphical user interfaces (GUIs) has made cryo-ET data processing more accessible to researchers without deep computational expertise. These interfaces simplify the complex steps involved in cryo-ET data analysis, from motion correction and tilt-series alignment to subtomogram averaging.
Deep Learning Algorithms for Particle Detection and Classification
Machine learning algorithms, such as deep learning, are used to automate particle picking and classify particles within complex tomograms. These algorithms enhance the speed and accuracy of particle detection, allowing for the identification of particles in crowded environments, such as within whole cells.
AI-Based Structure Prediction and Template Matching
AI-based methods also facilitate the process of template matching and structure prediction, where known structural models can be used to guide the refinement of the reconstructed 3D structures. This AI-driven approach accelerates the process of structure determination.
Software Tools and Packages for Cryo-ET Data Processing
Cryo-ET data processing involves several complex steps, including tilt-series alignment, motion correction, CTF estimation, tomogram reconstruction, and subtomogram averaging. To facilitate these processes, various software tools and packages have been developed, each with its own strengths and functionalities. Below, we will highlight some of the most widely used software tools in cryo-ET data processing, providing an overview of their features and capabilities.
| Category | Representative Software | Key Features |
|---|---|---|
| Tilt-Series Alignment | IMOD (4.11.4) | Gold fiducial tracking and geometric correction, supports multi-GPU acceleration. |
| Tomographic Reconstruction | TOM Toolbox, EMAN2 | Provides SIRT and MBIR (Model-Based Iterative Reconstruction) algorithms. |
| Subtomogram Averaging | RELION (3.1.2/4.0), PEET | Supports multi-class classification and missing wedge compensation; RELION 4.0 introduces VDAM to accelerate convergence. |
| Denoising & Segmentation | cryoCARE, ChimeraX | AI-driven noise modeling; ChimeraX enables automatic segmentation of complex structures (e.g., nuclear pore complexes). |
| Visualization & Modeling | UCSF Chimera, Dragonfly | Chimera offers multi-scale rendering, ideal for large assemblies like viral capsids. |
| Workflow Management | TOMOMAN, ScipionTomo | Integrates IMOD, RELION, and other tools for automated batch processing. |
1. AreTomo
AreTomo is a popular GPU-accelerated software package designed for the alignment and reconstruction of cryo-ET tilt-series. It is highly efficient and optimized for high-throughput processing, enabling rapid data analysis.
| Features |
|
| Strengths | The use of GPU technology allows AreTomo to deliver fast, accurate results, making it ideal for researchers working with large datasets in cryo-ET. |
2. Dynamo
Dynamo is a comprehensive software package for subtomogram averaging (STA) and 3D reconstruction in cryo-ET. It offers a flexible platform for analyzing tomographic data, with a strong focus on high-resolution reconstructions.
| Features |
|
| Strengths | Dynamo is widely used for cryo-ET data processing due to its robust capabilities in handling complex datasets and producing high-resolution structural models. |
3. RELION
Although originally developed for single-particle cryo-EM, RELION has been adapted for cryo-ET data processing, particularly for subtomogram averaging. It is one of the most well-known software packages in the cryo-EM community.
| Features |
|
| Strengths | RELION's comprehensive suite of tools makes it suitable for a wide range of cryo-ET data processing tasks, from particle detection to refinement, making it a versatile tool for structural biologists. |
4. EMRinger
EMRinger is a software tool used primarily for validation and refinement of protein structures obtained from cryo-ET and other cryo-EM techniques. It focuses on improving the accuracy of structural models by assessing geometry and stereochemistry.
| Features |
|
| Strengths | EMRinger is useful for ensuring that the final cryo-ET structures are accurate and fit within accepted structural conventions, especially for high-resolution models. |
5. Warp
Warp is an integrated software package designed to streamline the cryo-ET data processing workflow, combining multiple steps such as motion correction, tilt-series alignment, and subtomogram extraction into a unified interface.
| Features |
|
| Strengths | Warp's all-in-one platform and user-friendly interface make it a popular choice for cryo-ET practitioners looking for a simplified yet powerful data processing solution. |
6. Tomo3D
Tomo3D is a software package for the 3D reconstruction of tomographic data in cryo-ET. It is designed to handle large cryo-ET datasets and supports advanced data processing workflows.
| Features |
|
| Strengths | Tomo3D is favored for its robust capabilities in handling large datasets and its ability to deliver high-quality 3D reconstructions, even from noisy or incomplete data. |
7. DeepEM and CryoDRGN-ET
DeepEM and CryoDRGN-ET are deep learning-based tools designed to automate cryo-ET data processing, particularly in the areas of particle picking and classification.
| Features |
|
| Strengths | The use of deep learning algorithms allows for high-throughput and highly accurate particle detection, even in challenging, heterogeneous environments. |
Each of these software tools plays a crucial role in addressing different challenges in cryo-ET data processing, from data pre-processing and motion correction to subtomogram averaging and refinement. Depending on the specific needs of a study, researchers can choose the most appropriate software package to optimize their cryo-ET workflow and achieve the highest quality structural data.
Case Study: Cryo-ET Data Processing for Actin Filament Polarity Determination in Focal Adhesions
This case study focuses on the application of the Actin Polarity Toolbox (APT), a MATLAB-based tool, for processing cryo-ET data to determine actin filament polarity in focal adhesions (FAs).
Cryo-ET Data Processing Workflow
1. Data Acquisition and Preprocessing
- Sample Preparation: Mouse embryonic fibroblasts (MEFs) expressing vinculin-venus were cultured on EM grids. Cryo-correlated light and electron microscopy (Cryo-CLEM) was used to identify FAs, followed by cryo-ET imaging on a Titan Krios equipped with a Quantum energy filter and K2-Summit detector.
- Tilt-Series Collection: Tomograms were acquired at 42,000× magnification, a tilt range of −60° to +60°, and a defocus of −4 µm. The accumulated electron dose was ~75 e/Ų.
- Preprocessing: Motion correction was performed with MotionCorr, and CTF correction was applied using TOM Toolbox. Tomograms were reconstructed via weighted back-projection (WBP).
2. APT Workflow for Cryo-ET Data Processing
- Subtomogram Extraction: Actin filaments were manually segmented, and subtomograms were extracted.
- Alignment and Classification: RELION was used for 2D classification, filtering out false positives.
- 3D Reconstruction: A helical reconstruction approach was applied, achieving a resolution of 18.4 Å.
- Polarity Determination: The filament polarity was assessed using inverted psi angles (ψ⁻¹), with results showing two distinct peaks separated by 180°.
- Validation: Modeled tomograms were used to verify accuracy, with a filament error below 5%, even at low SNRs.
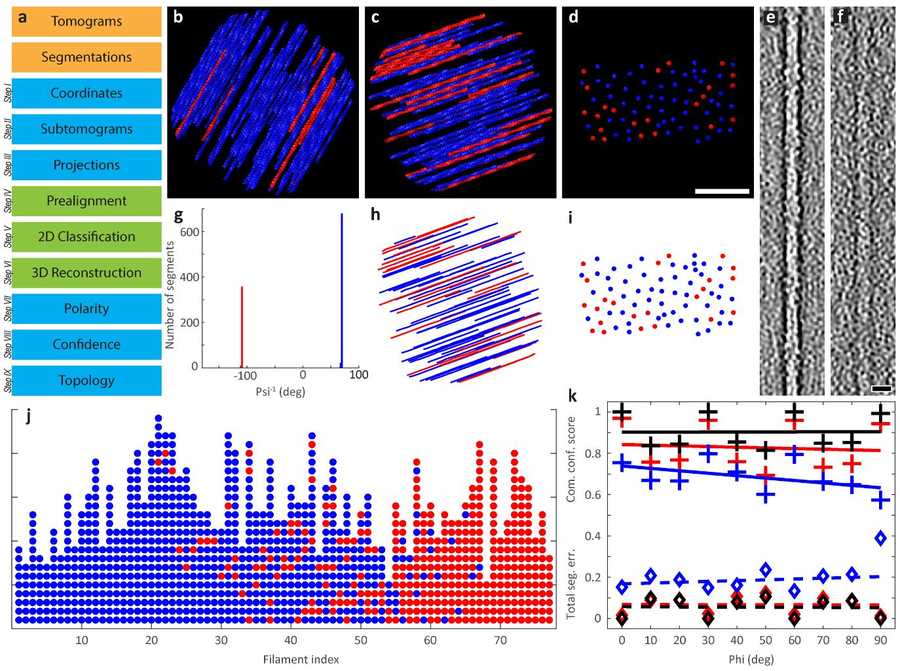 Figure 2. Polarity Determination of Modeled Actin Bundles. Workflow and validation of APT for actin bundle polarity determination. (a) Processing steps with MATLAB and RELION. (b-d) Top and side views of modeled actin bundles with varying tilt angles and filament orientations. (e-f) Effect of SNR on filament visualization. (Martins B, et al., 2021)
Figure 2. Polarity Determination of Modeled Actin Bundles. Workflow and validation of APT for actin bundle polarity determination. (a) Processing steps with MATLAB and RELION. (b-d) Top and side views of modeled actin bundles with varying tilt angles and filament orientations. (e-f) Effect of SNR on filament visualization. (Martins B, et al., 2021)
Key Findings
1. Actin Filament Reconstruction in FAs
Manual Segmentation: Seven tomograms were segmented, yielding 43,400 filament segments, with 20,585 selected for reconstruction at 18.4 Å resolution.
Automated Segmentation: A convolutional neural network (CNN) extended the dataset to 247,940 segments, with 72,973 used for reconstruction at 13.5 Å resolution.
2. Actin Polarity Distribution in FAs
Filament Analysis: 2,146 filaments were analyzed, totaling 149 µm in length, with an average filament length of ~70 nm.
Topology Mapping: Polarity transitioned from mixed near the FA base to uniform at distal regions, with most filaments oriented toward the cell tip.
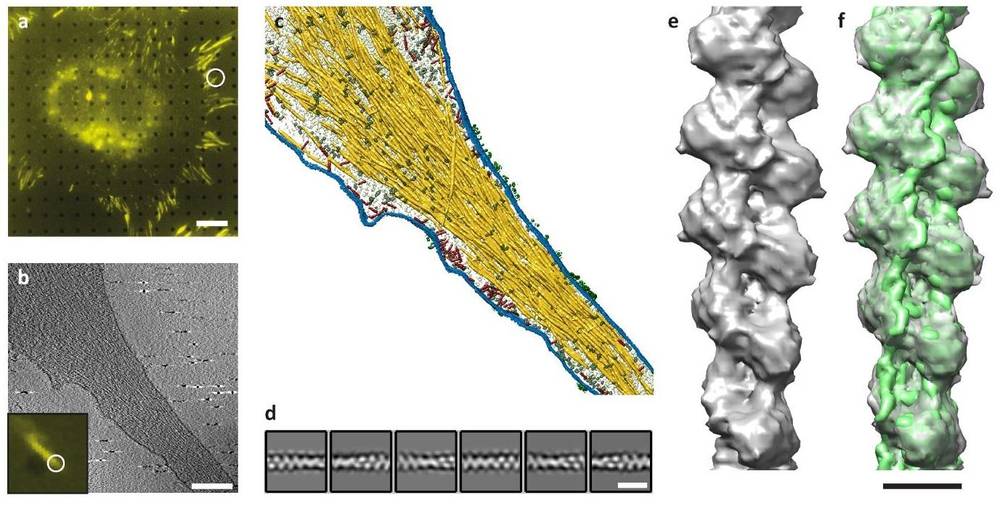 Figure 3. Cryo-Tomography of Focal Adhesions and Actin Filaments in Cells. (a) Fluorescence microscopy identifies focal adhesion (FA) sites. (b) Cryo-ET slice shows actin filaments and plasma membrane. (c) Surface rendering highlights actin (yellow), membranes (blue), macromolecules (red/grey), and receptor densities (green). (d) 2D class averages of actin filaments. (e) FA-associated actin filament structure. (f) Docking of in-vitro actin structure (EMD-6179) into in-situ data. (Martins B, et al., 2021)
Figure 3. Cryo-Tomography of Focal Adhesions and Actin Filaments in Cells. (a) Fluorescence microscopy identifies focal adhesion (FA) sites. (b) Cryo-ET slice shows actin filaments and plasma membrane. (c) Surface rendering highlights actin (yellow), membranes (blue), macromolecules (red/grey), and receptor densities (green). (d) 2D class averages of actin filaments. (e) FA-associated actin filament structure. (f) Docking of in-vitro actin structure (EMD-6179) into in-situ data. (Martins B, et al., 2021)
3. Correlation Between Polarity and FA Position
Topology Scores: Actin filament polarity showed a clear transition from mixed to uniform polarity along FA regions, correlating with FA position in protruding areas.
 Figure 4. Model of Actin Polarity at Focal Adhesions. Actin bundles in focal adhesions exhibit mixed polarity near proximal regions, transitioning to uniform polarity at distal regions. Filaments with plus-ends directed toward the cell body (red) surround a core of filaments pointing toward the cell periphery (blue). (Martins B, et al., 2021)
Figure 4. Model of Actin Polarity at Focal Adhesions. Actin bundles in focal adhesions exhibit mixed polarity near proximal regions, transitioning to uniform polarity at distal regions. Filaments with plus-ends directed toward the cell body (red) surround a core of filaments pointing toward the cell periphery (blue). (Martins B, et al., 2021)
This study demonstrates how APT effectively processes cryo-ET data to determine actin filament polarity within FAs. The workflow successfully addressed key data processing challenges, validated with ground truth models, and provided insights into the 3D architecture of actin networks. The results establish a clear link between actin polarity and FA positioning, highlighting the potential of cryo-ET combined with advanced data processing for studying cytoskeletal dynamics.
Cryo-ET data processing has undergone significant advancements in recent years, driven by innovations in sample preparation, data acquisition, and computational techniques. These improvements have made cryo-ET a more powerful and accessible tool, enabling researchers to answer complex biological questions that were once difficult to address. The integration of AI and machine learning holds promise for further enhancing cryo-ET's capabilities, making it an essential tool for structural biology.
For those looking to leverage cutting-edge cryo-ET service for structural analysis, Creative Biostructure offers comprehensive solutions to meet your research needs. Our expert team specializes in structural data processing and interpretation, providing tailored solutions to meet your project's needs. Contact us to explore how our cryo-ET and structural data analysis services can help you achieve breakthrough discoveries.
References
- Chen M, Bell J M, Shi X, et al. A complete data processing workflow for cryo-ET and subtomogram averaging. Nature Methods. 2019, 16(11): 1161-1168.
- Martins B, Sorrentino S, Chung W L, et al. Unveiling the polarity of actin filaments by cryo-electron tomography. Structure. 2021, 29(5): 488-498. e4.
- Pyle E, Zanetti G. Current data processing strategies for cryo-electron tomography and subtomogram averaging. Biochemical Journal. 2021, 478(10): 1827-1845.
- Watson A J I, Bartesaghi A. Advances in cryo-ET data processing: meeting the demands of visual proteomics. Current Opinion in Structural Biology. 2024, 87: 102861.
- Chen L, Fukata Y, Murata K. In situ cryo-electron tomography: a new method to elucidate cytoplasmic zoning at the molecular level. The Journal of Biochemistry. 2024, 175(2): 187-193.

-1.jpg)