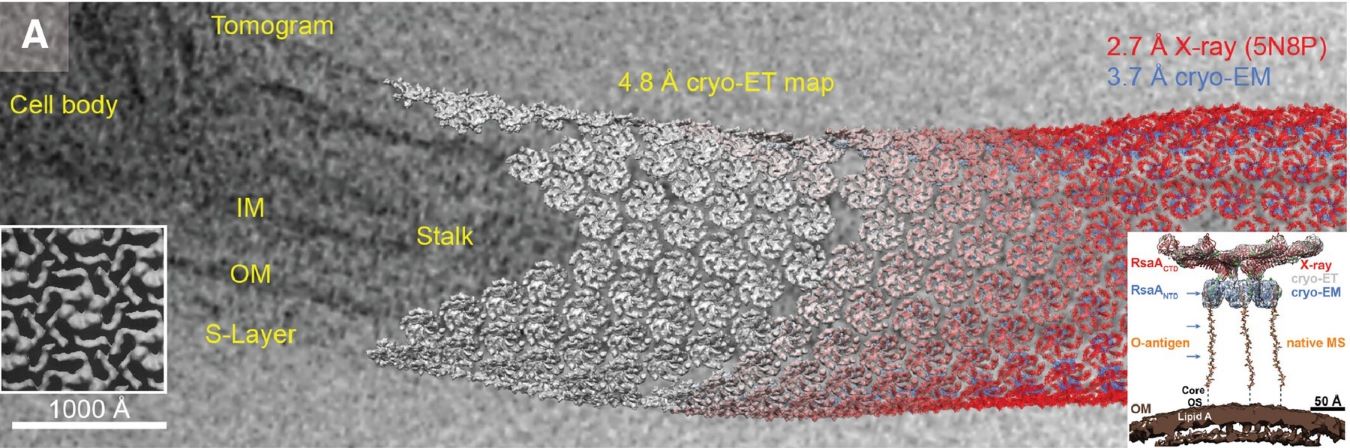
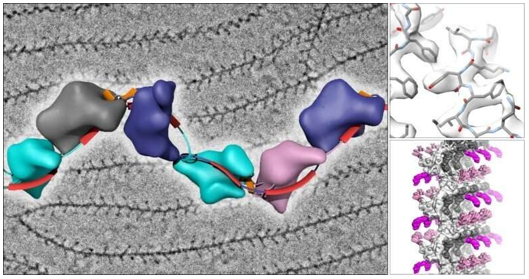
What Is Single Particle Cryo-EM
Cryo-EM single particle analysis (SPA) represents a groundbreaking structural biology technique that allows researchers to visualize and determine the three-dimensional structures of biological macromolecules in their near-native states at atomic or near-atomic resolution. Unlike traditional X-ray crystallography, this method doesn't require crystallization, making it particularly valuable for studying challenging targets such as membrane proteins, large protein complexes, and dynamic molecular assemblies.
Since its inception in the 1970s, cryo-EM has evolved dramatically through technological innovations, most notably the development of direct electron detectors and advanced computational algorithms. The 2013 breakthrough in achieving atomic resolution marked a revolutionary transformation in structural biology, enabling researchers to observe biological molecules in unprecedented detail and facilitating drug discovery by providing detailed insights into protein-drug interactions. This technique's ability to capture different conformational states of the same protein has made it an indispensable tool in modern structural biology and pharmaceutical research.
Basic Principles of Single Particle Cryo EM
Understanding Sample Preservation
The foundation of cryo-EM lies in its unique ability to preserve biological samples in their native state through vitrification. This process involves rapidly freezing the specimen in liquid ethane at temperatures around -190°C, creating a thin layer of amorphous ice that prevents the formation of crystalline ice, which could damage the specimen's structure. This preservation method allows proteins to maintain their natural conformations and functional states while protecting them from the high vacuum environment of the electron microscope.
Image Formation and Data Collection
In single-particle cryo-EM, a beam of electrons interacts with the frozen-hydrated specimen, generating two-dimensional projections of the molecules in various orientations. The sophisticated direct electron detectors capture these interactions with unprecedented sensitivity and speed, recording data as "movies" that can be corrected for beam-induced motion. This approach significantly improves the signal-to-noise ratio and resolution of the final reconstruction.
Computational Image Processing
The power of single-particle analysis lies in its computational approach to structure determination. Multiple images of individual particles are computationally aligned and classified to identify similar views. Advanced algorithms then combine thousands of particle images to reconstruct the three-dimensional structure, leveraging the random orientations of molecules in the ice to build a complete 3D model. This process effectively overcomes the limitation of low electron doses required to minimize radiation damage.
Advantages Over Traditional Structural Biology Methods
Cryo EM single particle analysis offers several distinct advantages compared to traditional structural biology techniques. Unlike X-ray crystallography, it doesn't require protein crystallization, making it ideal for studying challenging targets like membrane proteins and large complexes. The technique can capture multiple conformational states from a single sample, providing insights into protein dynamics. Additionally, it requires relatively small amounts of sample and can handle structural heterogeneity within the specimen.
Select Service
Single Particle Cryo-EM Workflow
1. Sample Preparation
Importance of Sample Quality
The success of cryo-EM analysis fundamentally depends on sample purity, stability, and concentration. High-quality samples should demonstrate biochemical homogeneity, minimal aggregation, and optimal protein concentration (typically 0.5-5 mg/mL). The sample's buffer composition, including pH, salt concentration, and additives, must be carefully optimized to maintain protein stability during the freezing process.
Methods for Vitrification and Grid Preparation
The vitrification process involves applying samples to specialized EM grids coated with holey carbon film. The grid is then blotted to achieve an optimal ice thickness (typically 50-100 nm) and rapidly plunged into liquid ethane cooled by liquid nitrogen. This ultra-rapid freezing creates vitreous ice, preserving the specimen in its native state.
2. Data Collection
Microscope Setup and Imaging Conditions
Data collection requires precise microscope alignment, including beam centering, astigmatism correction, and energy filter tuning. Key imaging parameters include acceleration voltage (typically 300kV), defocus range (-0.5 to -3.0 μm), and electron dose (40-60 e-/Å2). Direct electron detectors are operated in counting or super-resolution modes to optimize data quality.
Challenges in Data Collection
Major challenges include managing beam-induced motion, minimizing radiation damage, maintaining microscope stability, and ensuring consistent imaging conditions across thousands of micrographs. Ice thickness variations and specimen charging can also affect image quality.
3. Image Processing and Analysis
Particle Picking and 2D Classification
After motion correction and CTF estimation, particles are identified either automatically or semi-automatically from micrographs. Selected particles undergo 2D classification to group similar views and eliminate poor-quality particles. This step is crucial for improving the signal-to-noise ratio and assessing sample quality.
3D Reconstruction and Refinement
Initial 3D models are generated using either ab initio reconstruction or reference-based approaches. The model undergoes iterative refinement, incorporating particle orientation determination and 3D classification to handle structural heterogeneity.
Software Tools and Algorithms Used
Modern processing pipelines utilize specialized software packages including RELION, cryoSPARC, and EMAN2. These incorporate advanced algorithms such as maximum-likelihood approaches, Bayesian statistics, and neural networks for image processing and reconstruction.
4. Structural Interpretation and Validation
Resolution and Accuracy of Cryo-EM Structures
Resolution assessment primarily uses Fourier Shell Correlation (FSC) analysis, with the gold-standard FSC threshold at 0.143. Local resolution estimation helps identify regions of varying quality within the map. Map quality metrics include angular distribution analysis and tilt-pair validation.
Techniques for Validating Cryo-EM Models
Validation involves multiple approaches: cross-validation against independent data sets, analysis of model geometry and stereochemistry, assessment of fit between model and map, and comparison with existing structural and biochemical data. Independent map refinement and careful evaluation of conformational heterogeneity ensure reliable structure determination.
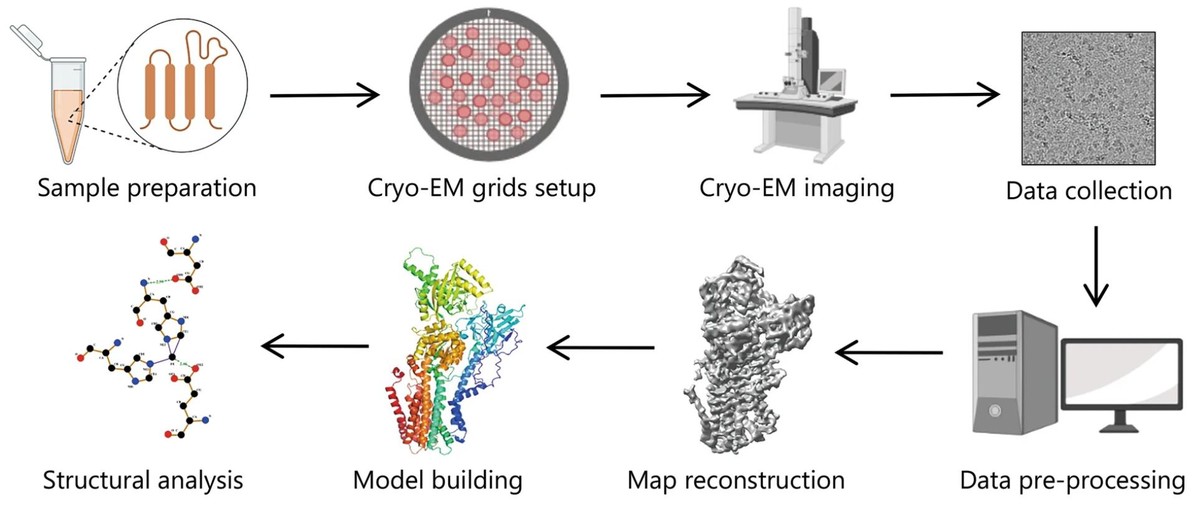 Figure 1. Single-Particle Cryo-EM Workflow for Structural Analysis. (Zhu K F, et al., 2023)
Figure 1. Single-Particle Cryo-EM Workflow for Structural Analysis. (Zhu K F, et al., 2023)
Applications of Single-Particle Cryo-EM
A. Single-Particle Cryo-EM of Biological Macromolecules
1. Examples of High-Resolution Structures
Cryo-EM has revolutionized our ability to visualize challenging biological targets at near-atomic resolution. Notable achievements include the determination of membrane protein structures such as ion channels (TRP channel family), large molecular machines like the spliceosome (>3MDa), and dynamic protein-protein complexes. The technique has been particularly successful in resolving structures previously inaccessible to traditional methods, including membrane proteins in lipid environments and flexible multi-domain proteins in different conformational states.
2. Impact on Understanding Molecular Mechanisms
These high-resolution structures have provided unprecedented insights into biological processes. For example, cryo-EM studies of ribosomes have revealed the detailed mechanics of protein synthesis, while analyses of ion channels have illuminated the molecular basis of ion transport and gating mechanisms. The ability to capture multiple conformational states has been crucial in understanding protein dynamics and regulatory mechanisms, providing a more complete picture of how proteins function in biological systems.
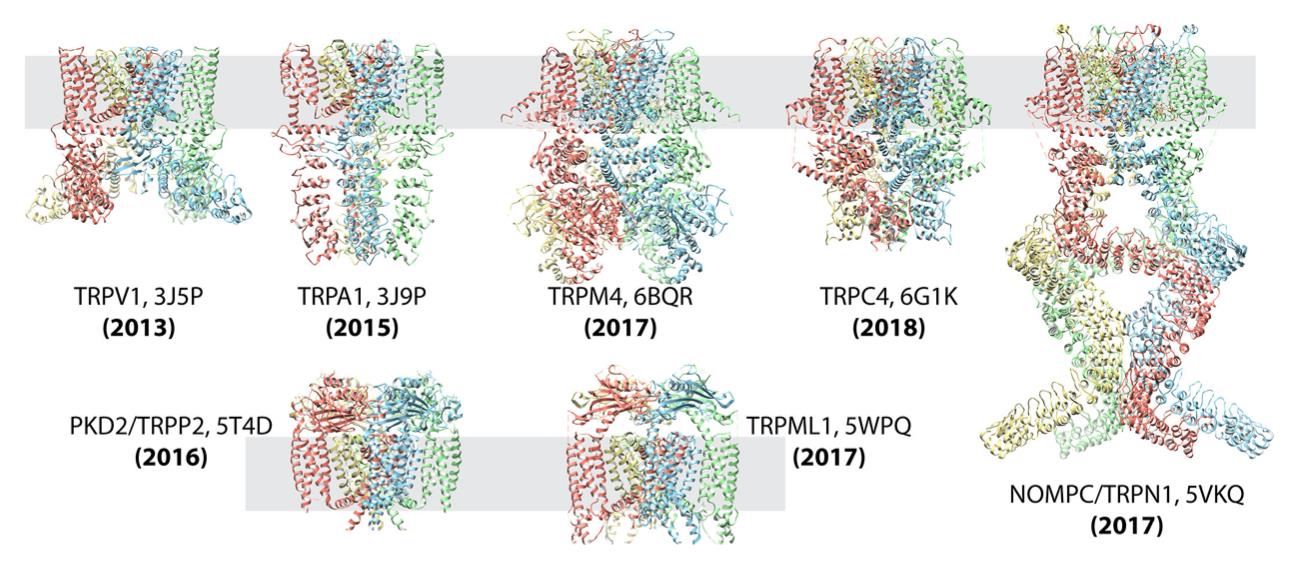 Figure 2. Single-Particle Cryo-EM Reveals TRP Channel Structures. Atomic structures of TRP channel subfamilies, including TRPV1, TRPA1, TRPM4, TRPC4, NOMOPC, PKD2, and TRPML, determined using single-particle cryo-EM. This technique has significantly accelerated membrane protein structural analysis. (Cheng Y, 2018)
Figure 2. Single-Particle Cryo-EM Reveals TRP Channel Structures. Atomic structures of TRP channel subfamilies, including TRPV1, TRPA1, TRPM4, TRPC4, NOMOPC, PKD2, and TRPML, determined using single-particle cryo-EM. This technique has significantly accelerated membrane protein structural analysis. (Cheng Y, 2018)
Select Service
- Cryo-EM for Membrane Proteins
- Cryo-EM for Protein Complex
- Cryo-EM for Protein-Ligand Complexes
- Cryo-EM for Small Proteins
- Cryo-EM for Ribosomes
- Cryo-EM for Viral Particle Identification and Characterization
- Cryo-EM for Bacteriophages
- Cryo-EM for DNA Samples
- Cryo-EM Analysis of High-resolution RNA Structures
- RNA Structure Analysis
B. Drug Discovery and Development
1. Role in Structure-Based Drug Design
Single-particle cryo-EM has emerged as a powerful tool in structure-based drug design by providing detailed views of drug binding sites and protein-ligand interactions. The technique's ability to reveal allosteric sites and conformational changes upon ligand binding has opened new opportunities for drug development. It particularly excels in studying membrane proteins and large complexes that are common drug targets but challenging to crystallize, enabling rational drug design for previously intractable targets.
A notable example is the development of oral small molecule agonists targeting GLP-1R (glucagon-like peptide-1 receptor). In this case, cryo-EM analysis of GLP-1R complexed with small molecules like PF-06883365 revealed key interaction sites with residues W33 and R380, providing crucial insights for drug optimization. This structural understanding helped develop PF-06882961, a promising oral therapeutic with high bioavailability.
2. Case Studies in Drug Target Identification
The method has been particularly successful with ion channels and receptors that were previously difficult to study. For instance, cryo-EM was instrumental in determining the structures of multiple TRP channel subfamily members within just five years, including TRPV1, TRPA1, TRPM4, TRPC4, TRPN1, PKD2, and TRPML. These structural insights have directly contributed to the development of targeted therapeutics for various conditions.
3. Applications in COVID-19 Drug Development
During the COVID-19 pandemic, cryo-EM played a crucial role in developing therapeutic antibodies. The development of Ambavirumab/Romisevirumab combination therapy was guided by cryo-EM structural analysis of the S-ACE2 complex. The structures revealed how these antibodies target different epitopes of the S protein, maintaining therapeutic activity against various mutant strains, including Omicron.
4. Impact on Drug Repurposing
Cryo-EM has also facilitated drug repurposing efforts. For example, structural studies of γ-secretase revealed why certain Alzheimer's disease drug candidates failed due to lack of specificity between APP and Notch proteolysis. This understanding led to the repurposing of nirogacestat for treating invasive fibroids, demonstrating how structural insights can guide the repositioning of existing drugs for new therapeutic applications.
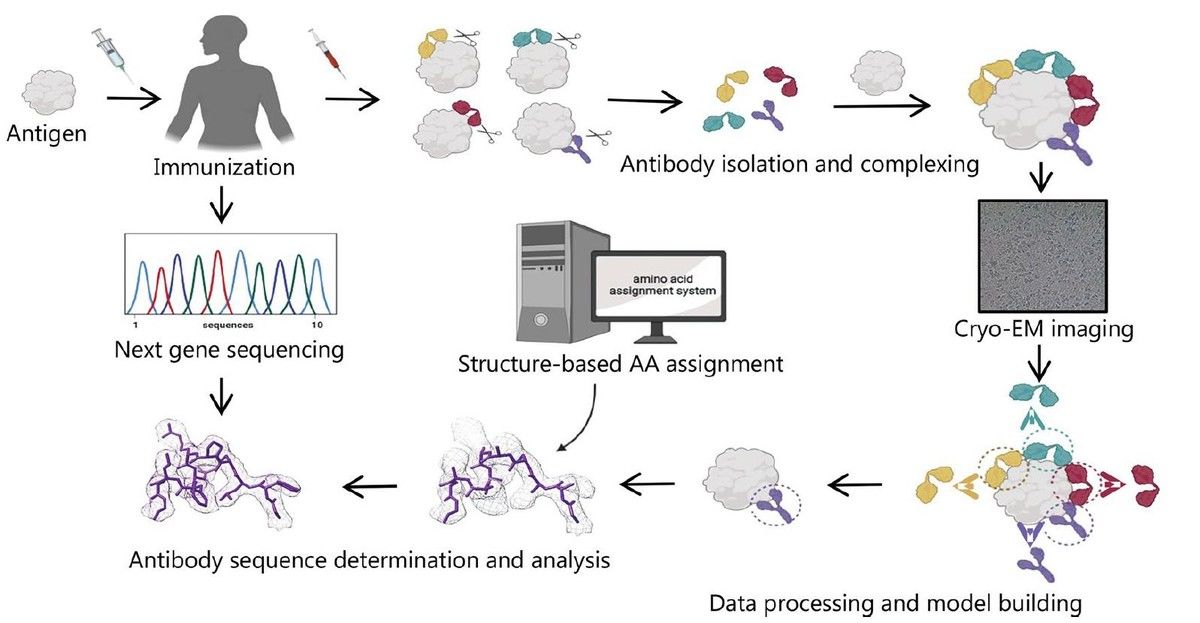 Figure 3. Cryo-EM and Sequencing-Based Antibody Discovery. (Zhu K F, et al., 2023)
Figure 3. Cryo-EM and Sequencing-Based Antibody Discovery. (Zhu K F, et al., 2023)
Select Service
- Cryo-EM for Drug Discovery
- Cryo-EM for Epitope Mapping
- Cryo-EM for Anticancer Research
- Cryo-EM for Antiviral Research
- Cryo-EM for RNA Transcription Regulation Analysis
- Cryo-EM for Structure-based Drug Design (SBDD)
- Cryo-EM for γ-Secretase
- Cryo-EM for GPCRs
- Cryo-EM for Ion Channels
- Cryo-EM for Antibodies
- Cryo-EM for E3-Ligases
- Cryo-EM for PROTAC
- Cryo-EM for Antigen-Antibody Complexes
Current Trends and Future Directions of Cryo-EM SPA
A. Technological Innovations
1. Advances in Microscope Hardware and Detectors
The field is witnessing rapid advancement in instrumentation, including the development of more stable microscopes with improved optics and superior electron sources. Next-generation direct electron detectors offer enhanced detection quantum efficiency (DQE) and faster frame rates, while innovations like the Volta phase plate are improving contrast for smaller proteins. Energy filters and aberration correctors are pushing the boundaries of achievable resolution and image quality.
2. Automation and High-Throughput Data Processing
Automated data collection systems and sophisticated image processing pipelines are transforming cryo-EM into a high-throughput technique. Machine learning algorithms are accelerating particle picking and classification, while GPU-accelerated computing is dramatically reducing processing times. Cloud-based platforms and integrated workflows are making the technique more accessible to the broader scientific community.
B. Challenges and Limitations
1. Radiation Damage and Signal-to-Noise Ratio
Despite technological advances, radiation damage remains a fundamental challenge, limiting the total electron dose that can be used for imaging. The inherent compromise between radiation damage and signal-to-noise ratio continues to affect achievable resolution, particularly for smaller proteins and dynamic regions. Current research focuses on developing methods to extract maximum information from minimal electron doses.
2. Theoretical and Practical Limits of Resolution
While theoretical limits suggest atomic resolution is achievable, practical barriers include beam-induced motion, sample preparation inconsistencies, and computational challenges in dealing with conformational heterogeneity. The field is actively working to overcome these limitations through improved sample preparation methods and more sophisticated data processing algorithms.
C. Future Prospects
1. Potential for Atomic-Resolution Structures
The future promises routine atomic resolution determination for an increasingly broad range of protein sizes and types. Emerging technologies suggest the possibility of reaching sub-2Å resolution more consistently, potentially revealing detailed chemical information about bound ligands and water molecules. This advancement could revolutionize our understanding of protein-drug interactions and catalytic mechanisms.
2. Integration with Other Structural Biology Techniques
The future of structural biology lies in hybrid approaches, combining cryo-EM with complementary techniques like X-ray crystallography, NMR spectroscopy, and mass spectrometry. Integration with time-resolved methods and in situ structural studies holds promise for understanding protein dynamics and function in more physiological contexts. These hybrid approaches will provide more complete pictures of biological processes across different spatial and temporal scales.
Single particle cryo-EM has evolved from a niche technique to a revolutionary tool in structural biology, offering atomic-resolution insights into complex biological systems. As this comprehensive overview demonstrates, the technology combines sophisticated sample preparation, advanced imaging capabilities, and powerful computational analysis to reveal molecular structures with unprecedented detail. At Creative Biostructure, we provide comprehensive cryo-EM services supported by experienced scientists and cutting-edge facilities. Whether you're investigating membrane proteins, macromolecular complexes, or dynamic molecular assemblies, our team is ready to help you unlock structural insights that can advance your research. Contact us to learn how our single particle cryo-EM expertise can support your research goals.
References
- Cheng Y, Grigorieff N, Penczek P A, et al. A primer to single-particle cryo-electron microscopy. Cell. 2015, 161(3): 438-449.
- Cheng Y. Single-particle cryo-EM—How did it get here and where will it go. Science. 2018, 361(6405): 876-880.
- Wrapp D, Wang N, Corbett K S, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020, 367(6483): 1260-1263.
- Nakane T, Kotecha A, Sente A, et al. Single-particle cryo-EM at atomic resolution. Nature. 2020, 587(7832): 152-156.
- Zhu K F, Yuan C, Du Y M, et al. Applications and prospects of cryo-EM in drug discovery. Military Medical Research. 2023, 10(1): 10.

-1.jpg)