What Is Cryo-EM Sample Preparation
Sample preparation for cryo EM is the gateway to revealing molecular structures at atomic resolution. This intricate process combines precise temperature control, specialized grid technology, and expert handling to transform biological specimens into analysis-ready samples. As the foundation of structural studies, the preparation quality directly determines the achievable resolution and reliability of structural data. Optimizing this critical step not only enhances data quality but also streamlines the entire structural analysis pipeline, offering a more efficient path to understanding complex molecular architectures. The strategic importance of proper sample preparation has grown alongside recent technological advancements in cryo-electron microscopy, making it a decisive factor in successful structural biology projects.
How to Prepare the Cryo EM Sample
1. Sample Optimization
The foundation of successful Cryo-EM analysis begins with meticulous sample optimization. This process requires careful consideration of protein concentration, typically ranging from 0.5 to 5 mg/mL, depending on the molecular weight and shape of the target. Buffer composition plays a crucial role, where optimal ionic strength and pH create a stable environment that prevents particle aggregation while maintaining biological relevance. Sample homogeneity assessment through techniques like dynamic light scattering helps identify the ideal preparation conditions, ensuring uniform particle distribution essential for high-resolution structural determination.
2. Grid Selection and Preparation
The choice and preparation of EM grids significantly influence imaging quality and data collection efficiency. Modern carbon-based support films, featuring precisely engineered hole patterns, provide optimal balance between mechanical stability and imaging areas. Surface treatment protocols, including glow discharge or plasma cleaning, modify grid hydrophilicity to ensure proper sample spreading. The hole size selection depends on particle dimensions, with typical ranges from 1.2 to 2 μm offering versatility for various biological specimens. Clean handling procedures and environmental control during grid preparation prevent contamination that could compromise data quality.
3. Vitrification Process
The transformation of liquid samples into vitreous ice represents a critical moment in sample preparation. This rapid freezing process, occurring within milliseconds, must achieve cooling rates exceeding 10,000°C per second to prevent crystalline ice formation. Environmental parameters, including chamber humidity and temperature, require precise control to ensure reproducible results. The plunge-freezing depth and speed must be optimized based on sample characteristics, while the time between sample application and freezing needs careful management to prevent unwanted sample concentration effects or degradation.
Each step demands careful attention to detail and understanding of the specific requirements for different biological systems. Success in Cryo-EM sample preparation relies on the synergy between these components, where optimization of one element often influences the parameters of others.
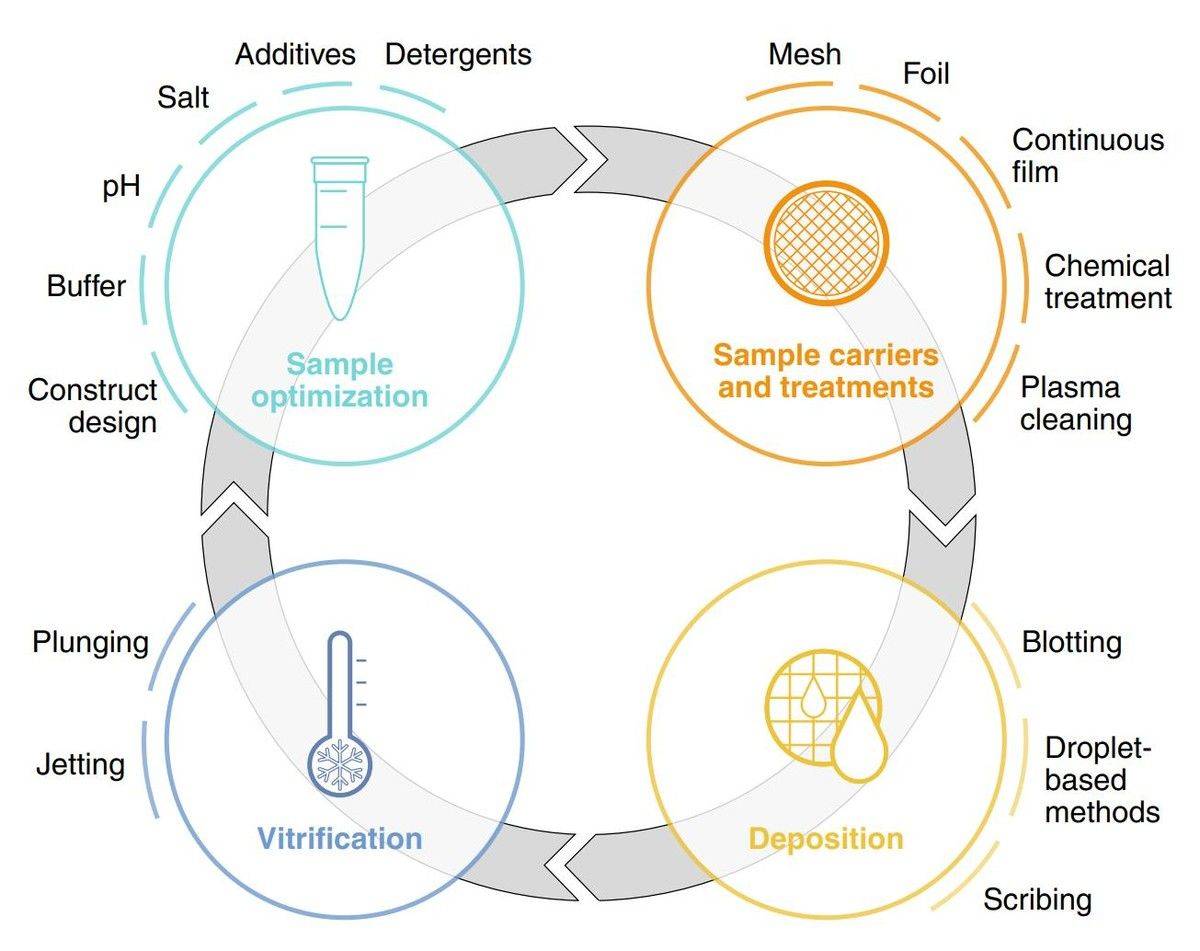 Figure 1. An overview of the key stages in cryo-EM sample preparation. (Weissenberger G, et al., 2021)
Figure 1. An overview of the key stages in cryo-EM sample preparation. (Weissenberger G, et al., 2021)
Advanced Techniques in Cryo EM Sample Preparation
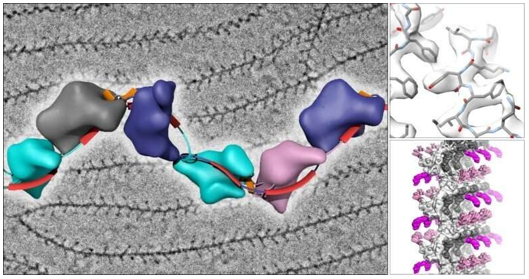
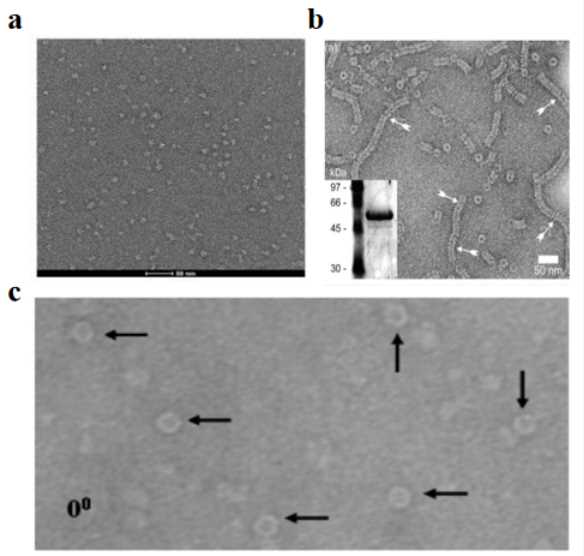
A. Single Particle Analysis Sample Preparation
Single particle analysis requires distinct preparation strategies to achieve optimal particle distribution and orientation. The technique demands precise control of surface tension and droplet spreading to achieve uniform particle distribution. Sample concentration optimization typically follows a systematic approach, starting with dilution series to identify conditions that yield 2-3 particles per hole. For membrane proteins, the addition of specific detergents or nanodiscs during preparation helps maintain structural integrity while preventing aggregation. The incorporation of gentle crosslinking agents can stabilize dynamic complexes without compromising their native structure, particularly beneficial for large molecular assemblies.
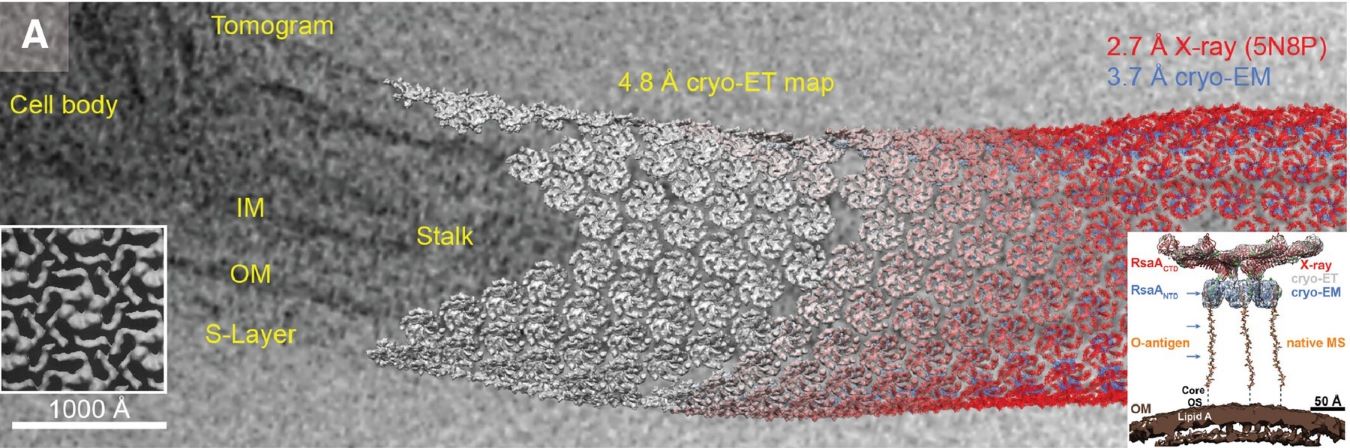
B. Cryo-Electron Tomography Sample Preparation
Cryo-ET sample preparation introduces unique challenges due to specimen thickness requirements. The process involves careful thinning of cellular samples to achieve electron-transparent regions, typically 100-200 nm thick. For cellular specimens, focused ion beam milling has emerged as a crucial step, requiring precise control of milling parameters to prevent sample damage. The addition of fiducial gold markers during preparation facilitates subsequent alignment of tilt series. Grid preparation often incorporates back-side blotting techniques to preserve delicate cellular structures, while maintaining proper ice thickness across the imaging area.
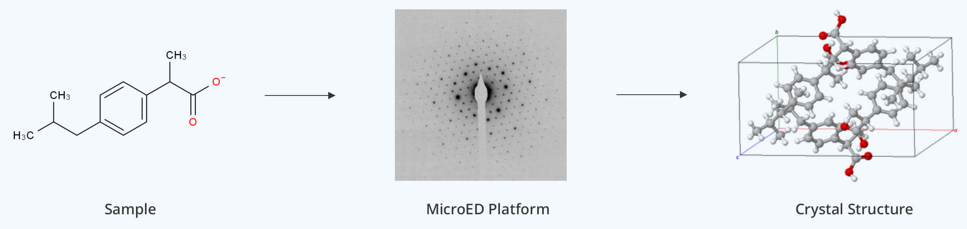
C. Microcrystal Electron Diffraction Sample Preparation
MicroED preparation techniques focus on achieving optimal crystal size and orientation. The method requires specialized approaches for handling microcrystals, typically ranging from 0.1-1 μm in thickness. Sample preparation often involves gentle crushing of larger crystals or controlled crystallization to obtain suitable microcrystals. The process demands careful control of crystal density on the grid to prevent overlapping while ensuring sufficient sampling. Humidity control during transfer becomes particularly critical to prevent crystal deterioration. The technique often employs specialized grid coating procedures to enhance crystal adherence while maintaining their diffraction quality.
Each of these advanced preparation methods requires specific optimization based on:
- Sample characteristics and stability
- Required resolution targets
- Data collection strategy
- Downstream processing requirements
The success of these techniques relies heavily on the precise control of environmental conditions and the careful selection of preparation parameters to match specific experimental goals.
Select Service
Optimization Strategies for Different Sample Types
A. Cryo EM Protein Sample Preparation
Successful protein sample preparation hinges on maintaining structural integrity throughout the process. Initial sample quality assessment should verify monodispersity through size exclusion chromatography. Buffer optimization plays a critical role - reducing salt concentration to 150-200 mM typically enhances contrast, while the addition of specific stabilizers like glycerol (removed just before vitrification) can protect against air-water interface damage. For membrane proteins, detergent screening becomes essential, with common strategies including systematic testing of different detergent types and concentrations. The final pre-vitrification steps often incorporate limited proteolysis or chemical crosslinking to stabilize specific conformational states.
B. Cellular Specimens
Cellular sample preparation requires balancing structural preservation with electron transparency. Pre-treatment protocols often include careful cell density optimization to prevent overcrowding while ensuring sufficient sampling areas. The incorporation of cryoprotectants requires precise timing to prevent osmotic shock while maintaining cellular architecture. Adherent cells benefit from direct growth on EM grids with specialized surface treatments, while suspension cells need careful concentration adjustments before application. Time management becomes crucial during preparation to minimize cellular stress responses that could alter native structures.
C. Macromolecular Complexes
Large molecular assemblies demand specialized approaches due to their size and structural complexity. Initial stability assessment through analytical ultracentrifugation helps determine optimal buffer conditions. The preparation process often requires careful adjustment of complex concentration to prevent dissociation while maintaining sufficient particle density. For particularly unstable complexes, chemical crosslinking protocols using graduated fixative concentrations can help preserve quaternary structure. Grid surface modification strategies, including the use of thin continuous carbon films or specific functional groups, can help prevent complex dissociation during sample application and blotting.
Key Optimization Parameters Across Sample Types:
- Temperature control during all preparation steps
- Blotting force and duration optimization
- Ice thickness management
- Time intervals between preparation steps
- Environmental humidity control
Each sample type requires specific attention to these parameters, with successful optimization often involving systematic testing and careful documentation of outcomes for reproducibility.
Select Service
- Cryo-EM for Membrane Proteins
- Cryo-EM for Protein Complex
- Cryo-EM for Protein-Ligand Complexes
- Cryo-EM for Small Proteins
- Cryo-EM for Ribosomes
- Cryo-EM for Viral Particle Identification and Characterization
- Cryo-EM for Bacteriophages
- Cryo-EM for DNA Samples
- Cryo-EM Analysis of High-resolution RNA Structures
- RNA Structure Analysis
- Cryo-EM for Subcellular Structure Analysis
Quality Control in Cryo-EM Sample Preparation
Assessment Methods
Quality assessment begins with systematic screening of grid preparation conditions. Initial screening using low-magnification imaging helps evaluate ice distribution and thickness uniformity across grid squares. Direct assessment of particle distribution through real-time imaging provides immediate feedback on sample concentration and spreading behavior. Advanced screening protocols incorporate automated hole mapping to identify optimal imaging areas, maximizing data collection efficiency. Documentation of environmental parameters during preparation, including humidity levels and blotting conditions, enables correlation between preparation conditions and sample quality.
Validation Techniques
Sample validation employs multiple complementary approaches to ensure preparation quality. Preliminary evaluation using negative staining helps verify sample integrity before proceeding to cryo-conditions. Real-time ice quality assessment through electron diffraction patterns reveals crystalline ice contamination or other vitrification issues. Particle picking from initial micrographs provides crucial information about orientation distribution and aggregation state. Advanced validation includes:
- 2D classification of preliminary datasets
- Assessment of contrast transfer function (CTF) fitting quality
- Evaluation of background noise levels
- Analysis of particle size distribution patterns
Quality Standards
Industry-standard quality metrics ensure reproducible and reliable sample preparation. Key quality indicators include:
- Ice thickness uniformity across grid areas
- Particle density distribution within acceptable ranges
- Absence of contamination or crystalline ice
- Consistent particle orientation distribution
- Sample stability during extended imaging sessions
The development of robust quality control measures significantly impacts downstream processing success and ultimately determines the achievable resolution in structural studies.
Troubleshooting Guide for Cryo-EM Sample Preparation
| Issue Category | Problem Description | Solutions | Preventive Measures |
| Ice Quality | Non-uniform ice thickness or crystalline ice formation |
|
|
| Sample Distribution | Uneven particle distribution or empty grid areas |
|
|
| Particle Aggregation | Sample clustering or preferred orientation |
|
|
| Contamination | Surface contaminants or sample degradation |
|
|
| Data Collection | Poor contrast or insufficient views |
|
|
This table provides a structured approach to identifying and resolving common issues in Cryo-EM sample preparation, enabling efficient troubleshooting and problem prevention.
Cryo EM Sample Prep Emerging Technologies
Advanced Grid Technologies
Latest innovations in grid design are transforming sample preparation workflows. New developments include:
- Self-wicking Grids: Engineered surface properties enable automatic sample distribution, reducing operator variability while improving reproducibility.
- Metallic Alloy Supports: Advanced materials provide superior thermal conductivity and mechanical stability during sample preparation.
- Patterned Substrates: Specialized surface modifications guide particle orientation and distribution for enhanced data quality.
- Hybrid Support Films: Novel combinations of materials offer improved sample stability while maintaining high contrast.
Automated Cryo-EM Sample Preparation
Automated systems are revolutionizing sample preparation throughput and consistency. Key developments include:
- Environmental Control: Integrated chambers maintain precise humidity and temperature conditions throughout preparation.
- Robotic Grid Handling: Automated systems minimize human contact and ensure reproducible sample application.
- Real-time Monitoring: Advanced sensors provide immediate feedback on preparation quality.
- Data Management: Automated logging systems ensure complete preparation parameter documentation.
- AI-assisted Optimization: Machine learning algorithms help identify optimal preparation conditions.
Novel Vitrification Methods
New approaches to sample vitrification are expanding preparation capabilities. Innovations include:
- Spray-based Systems: Alternative sample delivery methods reduce sample consumption and improve vitrification speed.
- Time-resolved Preparation: Advanced techniques enable capture of dynamic molecular states.
- Alternative Cooling: Novel approaches to achieve vitreous ice formation while minimizing sample damage.
- Integrated Analytics: Real-time sample characterization during preparation process.
- Temperature Control: Precision systems enable better control over vitrification conditions.
Industry Impact and Future Outlook
Integration of emerging technologies is transforming structural biology research. Key benefits include:
- Accelerated Discovery: Streamlined workflows enable faster structural determination.
- Enhanced Reliability: Improved reproducibility in sample preparation.
- Broader Applications: Extended capabilities for challenging sample types.
- Cost Efficiency: Reduced sample consumption and improved success rates.
- Quality Improvement: Better structural preservation and data quality.
Understanding and mastering Cryo-EM sample preparation is crucial for successful structural biology research. This comprehensive overview demonstrates the complexity and precision required at each step, from initial sample optimization to final vitrification. The continuous advancement of preparation techniques, coupled with emerging technologies, opens new possibilities for structural studies across diverse biological systems.
At Creative Biostructure, we offer comprehensive cryo-EM services with expertise in handling diverse sample types, from protein complexes to cellular specimens. Our experienced team utilizes state-of-the-art facilities and advanced preparation techniques to ensure optimal results for your structural biology projects. Contact us to discuss how our Cryo-EM sample preparation expertise can advance your research goals.
References
- Thompson R F, Walker M, Siebert C A, et al. An introduction to sample preparation and imaging by cryo-electron microscopy for structural biology. Methods. 2016, 100: 3-15.
- Passmore L A, Russo C J. Specimen preparation for high-resolution cryo-EM. Methods in Enzymology. 2016, 579: 51-86.
- Weissenberger G, Henderikx R J M, Peters P J. Understanding the invisible hands of sample preparation for cryo-EM. Nature Methods. 2021, 18(5): 463-471.
- Vénien-Bryan C, Fernandes C A H. Overview of membrane protein sample preparation for single-particle cryo-electron microscopy analysis. International Journal of Molecular Sciences. 2023, 24(19): 14785.
- Klebl D P, Aspinall L, Muench S P. Time resolved applications for Cryo-EM; approaches, challenges and future directions. Current Opinion in Structural Biology. 2023, 83: 102696.

-1.jpg)