Brief Introduction to Cryo-EM
Cryo-electron microscopy (Cryo-EM) represents a groundbreaking advancement in structural biology, allowing scientists to visualize biological molecules at near-atomic resolution while maintaining them in their native states. This revolutionary technique, which earned Jacques Dubochet, Joachim Frank, and Richard Henderson the 2017 Nobel Prize in Chemistry, involves flash-freezing biological samples in a thin layer of vitreous ice and imaging them using electron microscopy. Unlike traditional methods such as X-ray crystallography, Cryo-EM can analyze complex molecular structures that are difficult or impossible to crystallize, including large protein complexes, membrane proteins, and dynamic molecular assemblies. The technique has evolved from generating low-resolution "blob-like" images in the 1980s to achieving atomic-level resolution today, fundamentally transforming our understanding of cellular machinery and accelerating drug discovery processes.
How Cryo-EM is Revolutionizing Structural Biology
A. High-Resolution Structure Determination
Advances in Resolution
Recent breakthroughs in cryo-EM technology have enabled unprecedented achievement of sub-2Å resolution for complex biological structures, reaching atomic-level precision. This dramatic improvement is driven by innovations in direct electron detectors, sophisticated motion correction algorithms, and enhanced image processing techniques. These advances now allow researchers to visualize individual atoms within intricate biological molecules, providing crucial insights into molecular mechanisms that were previously unattainable.
Comparison with Traditional Methods
While X-ray crystallography and NMR spectroscopy have been foundational techniques in structural biology, cryo-EM offers distinct advantages. Unlike crystallography, which requires sample crystallization that can potentially alter native conformations, cryo-EM enables visualization of molecules in their natural states. Additionally, cryo-EM surpasses size limitations of NMR, accommodating larger molecular complexes, and requires significantly less sample material - typically in the microgram range compared to milligrams needed for traditional methods.
B. Overcoming Challenges in Sample Preparation
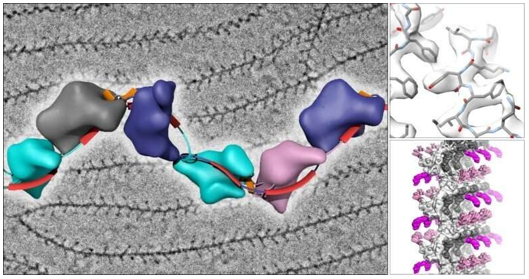
RNA Structure Determination
Cryo-EM has revolutionized RNA structural biology by enabling the direct visualization of RNA molecules in their native conformational states. Through advanced vitrification techniques and scaffolding approaches, researchers can now capture and analyze dynamic RNA structures with unprecedented detail, revealing complex folding patterns and functional mechanisms that were previously inaccessible.
Protein-Free RNA Structures
A major breakthrough in cryo-EM applications is the ability to determine structures of protein-free RNAs at near-atomic resolution. Using innovative scaffolding strategies, researchers can now visualize small to moderate-sized RNAs (from 86 to 210 nucleotides) at resolutions as high as 2.5Å, enabling detailed understanding of RNA folding, catalytic mechanisms, and regulatory functions.
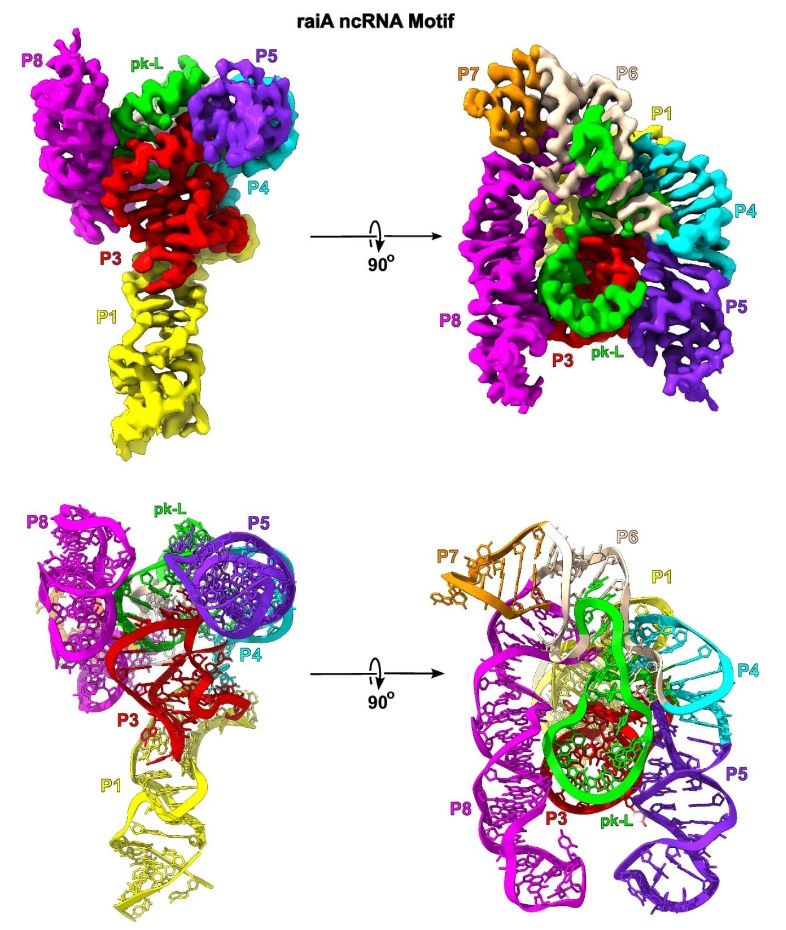 Figure 1. Cryo-EM Map and Model of Scaffolded raiA Non-Coding RNA. (Haack D B, et al., 2024)
Figure 1. Cryo-EM Map and Model of Scaffolded raiA Non-Coding RNA. (Haack D B, et al., 2024)
Select Service
Related Reading
C. Impact on Drug Development
Structure-Based Drug Design
Cryo-EM has transformed drug discovery by providing detailed structural information about drug targets in their native states at atomic resolution. This capability enables rational drug design through precise visualization of ligand-protein interactions, water networks in binding sites, and conformational changes upon drug binding, leading to more efficient therapeutic development processes.
Case Study: Human CDK-Activating Kinase
The determination of the human CDK-activating kinase structure through cryo-EM exemplifies its impact on drug development. Recent studies have achieved resolutions up to 1.8Å for the CDK complex bound to various inhibitors, revealing crucial details about binding mechanisms and water-mediated interactions. This structural information has directly facilitated the development of selective inhibitors, demonstrating the power of cryo-EM in structure-based drug design.
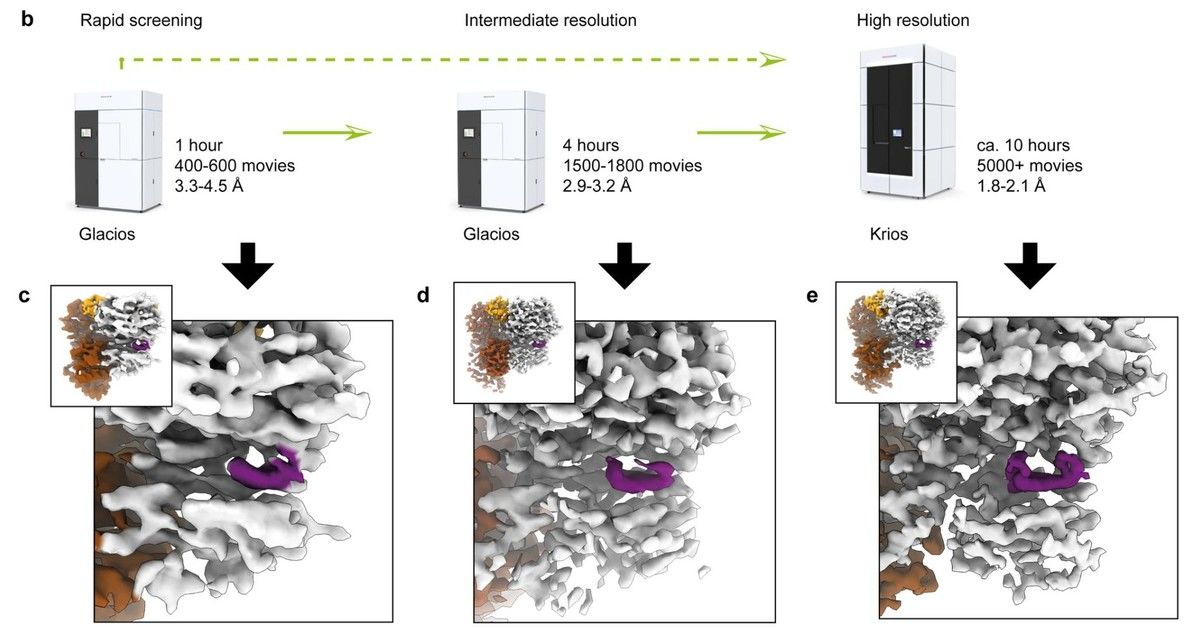 Figure 2. Tripartite Cryo-EM Workflow and Inhibitor Screening Results. (Cushing V I, et al., 2024)
Figure 2. Tripartite Cryo-EM Workflow and Inhibitor Screening Results. (Cushing V I, et al., 2024)
Applications of Cryo-EM in Structural Biology
A. Protein Structures
Cryo-EM has emerged as a powerful technique for determining protein structures across diverse scales and complexities, from small individual proteins to large macromolecular assemblies. Its ability to capture proteins in their native states and reveal multiple conformational states has provided unprecedented insights into protein function and regulation, making it an invaluable tool for both basic research and drug development.
High-Resolution Protein Mapping
Cryo-EM has enabled detailed mapping of protein structures at atomic resolution, revealing subtle conformational changes and dynamic states that were previously inaccessible. This capability has provided unprecedented insights into protein function and regulation mechanisms.
Complex Protein Structures
The technique has proven particularly powerful in analyzing large protein complexes and membrane proteins that resist crystallization. Cryo-EM can capture these structures in their native states, revealing dynamic assemblies and transient interactions critical for cellular function, while preserving delicate structural features that might be lost in crystal packing.
B. Viral Structures
Cryo-EM has revolutionized our understanding of viral architecture and infection mechanisms by enabling direct visualization of viral particles in their native states at unprecedented resolutions. This technological breakthrough has proven particularly valuable for rapid structural characterization of emerging viral pathogens and facilitating the development of targeted therapeutics and vaccines.
Cryo-EM Structure of Zika Virus
Cryo-EM has provided near-atomic resolution structural analysis of the Zika virus, uncovering detailed insights into its capsid assembly and envelope proteins. This information has deepened our understanding of virus-host interactions, identified potential therapeutic targets, and advanced vaccine development.
The 3.8Å cryo-EM structure of the mature Zika virus revealed the arrangement of 180 envelope glycoproteins forming the icosahedral shell and highlighted unique features near the Asn154 glycosylation site, distinct from other flaviviruses. This site, visible in the cryo-EM density, likely plays a crucial role in host cell binding and entry, potentially influencing virus transmission and disease.
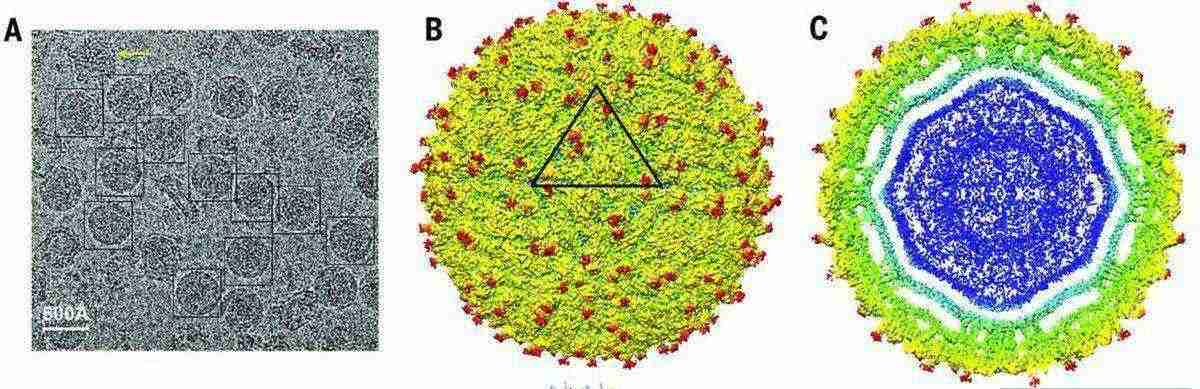 Figure 3. Cryo-EM Analysis of Zika Virus (ZIKV) Structure. (Sirohi D, et al., 2016)
Figure 3. Cryo-EM Analysis of Zika Virus (ZIKV) Structure. (Sirohi D, et al., 2016)
2019-nCoV Spike Protein
High resolution cryo EM analysis of the SARS-CoV-2 spike protein has been pivotal in understanding viral entry and host receptor recognition, directly aiding vaccine development and therapeutic antibody design.
The 3.5Å structure of the spike (S) glycoprotein in its prefusion conformation revealed that the trimer predominantly exists with one receptor-binding domain (RBD) in an up position, allowing receptor access. It also showed that the SARS-CoV-2 S protein binds human ACE2 with higher affinity than the SARS-CoV S protein, explaining its enhanced transmissibility. These insights have accelerated the development of medical countermeasures during the pandemic.
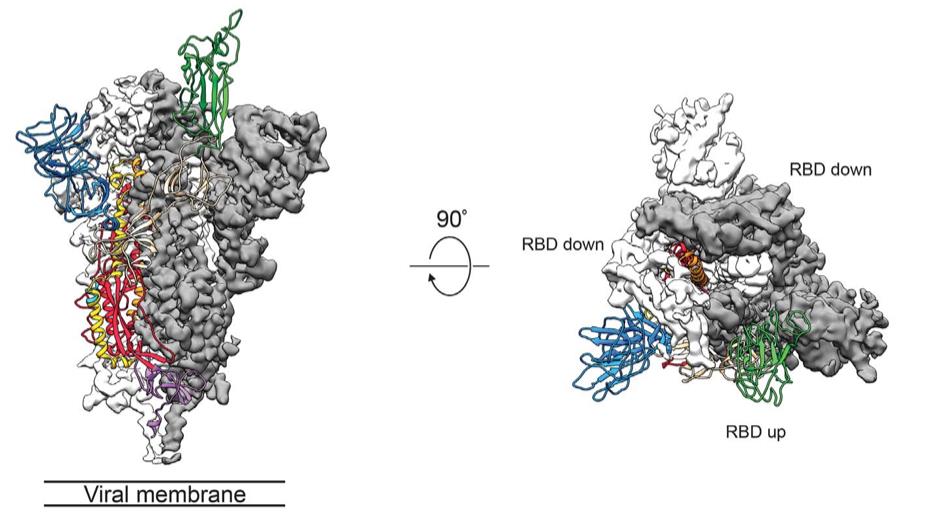 Figure 4. Cryo-EM structure of the 2019- nCoV spike in the prefusion conformation. (Wrapp D, et al., 2020)
Figure 4. Cryo-EM structure of the 2019- nCoV spike in the prefusion conformation. (Wrapp D, et al., 2020)
C. Ribosome Structures
Cryo-EM has transformed our understanding of protein translation by enabling visualization of ribosomes in multiple functional states at near-atomic resolution. This breakthrough has been particularly significant because it allows researchers to observe dynamic conformational changes during translation, providing unprecedented insights into both fundamental biological processes and disease mechanisms related to protein synthesis.
High-Resolution Ribosome Imaging
Advanced cryo-EM techniques have enabled visualization of ribosome structures at unprecedented resolution levels, revealing intricate details of ribosomal RNA and protein interactions. These studies have captured various functional states of the ribosome, providing detailed snapshots of the translation machinery in action.
Implications for Translation Studies
Structural insights from cryo-EM have revolutionized our understanding of protein synthesis mechanisms. The ability to visualize different conformational states has illuminated critical aspects of translation initiation, elongation, and termination, leading to new understanding of translational regulation and disease mechanisms.
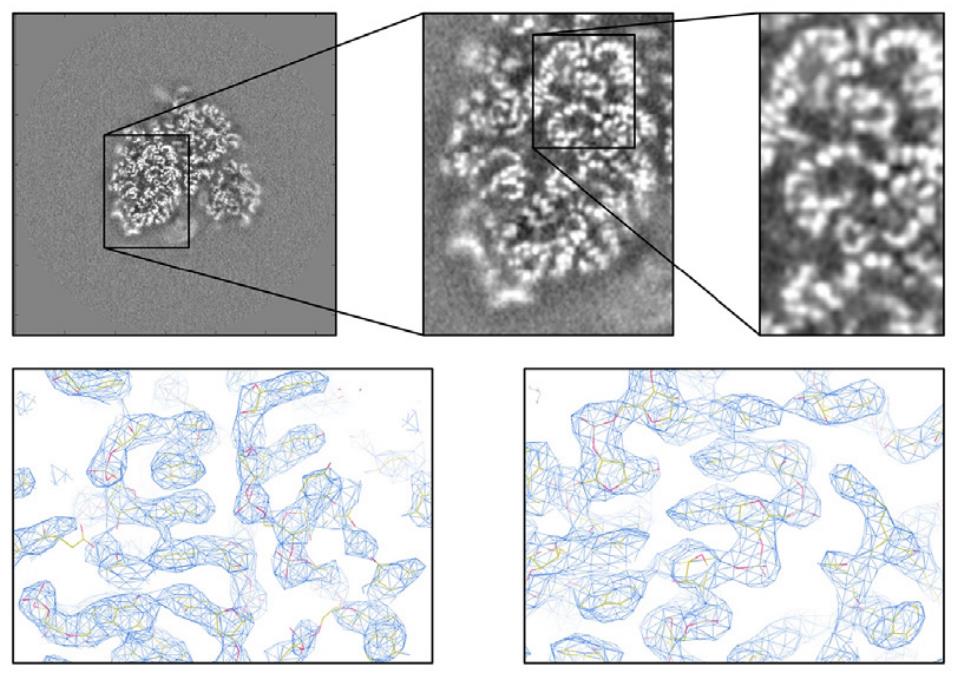 Figure 5. Cryo-EM Analysis of Ribosomal RNA and Refinement Enhancements. Top: Cryo-EM map slices revealing structural details, including the phosphate backbone of ribosomal RNA. Bottom: Enhanced cryo-EM reconstructions of the human ribosome achieved through focused refinements applied to individual subunits, improving resolution and structural clarity. (von Loeffelholz O, et al., 2017)
Figure 5. Cryo-EM Analysis of Ribosomal RNA and Refinement Enhancements. Top: Cryo-EM map slices revealing structural details, including the phosphate backbone of ribosomal RNA. Bottom: Enhanced cryo-EM reconstructions of the human ribosome achieved through focused refinements applied to individual subunits, improving resolution and structural clarity. (von Loeffelholz O, et al., 2017)
D. Neurodegenerative Diseases
The application of cryo-EM in studying neurodegenerative diseases has provided groundbreaking insights into the molecular basis of these conditions. By enabling high-resolution visualization of disease-specific protein aggregates and their interactions with potential therapeutic compounds, cryo-EM has become an indispensable tool in understanding disease mechanisms and developing targeted treatments.
Cryo-EM Structures of tau Filaments from Alzheimer's Disease
Cryo-EM has become an essential tool for studying the molecular pathology of neurodegenerative diseases such as Alzheimer's disease. It enables the high-resolution visualization of tau filaments, a hallmark of the disease, providing detailed insights into their structures. This allows researchers to uncover how tau proteins misfold and aggregate into paired-helical filaments (PHFs) and straight filaments, a process central to disease progression.
PET Ligand Binding
High-resolution cryo EM structures has significantly advanced the development of positron emission tomography (PET) ligands, which are critical for diagnosing and monitoring Alzheimer's disease. By resolving the binding interactions of second-generation PET ligands, such as MK-6240, Cryo-EM reveals how these molecules interact with specific residues in the tau PHF structure. For instance, the binding of MK-6240 at a 1:1 ratio within the cleft of tau PHF, engaging residues like glutamine 351 and lysine 353, has been visualized at near-atomic resolution. This structural information not only explains the mechanism of PET ligand specificity but also facilitates the rational design of more effective imaging agents and potential therapeutics targeting tau amyloids.
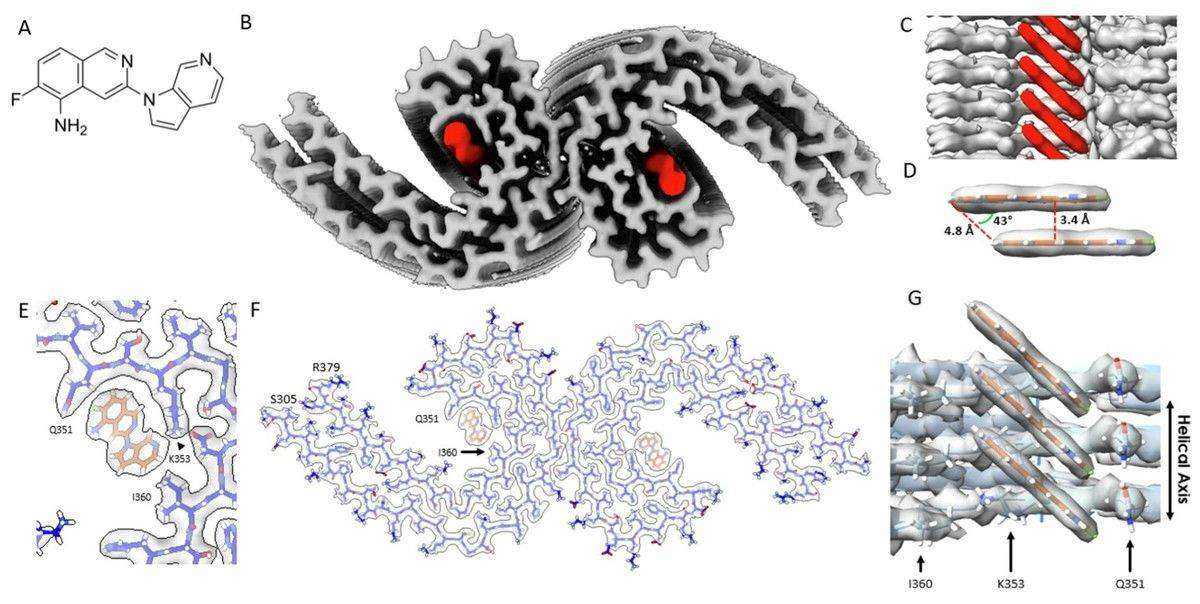 Figure 6. Cryo-EM Structure of MK-6240 Bound to Tau Paired Helical Filaments. (Kunach P, et al., 2024)
Figure 6. Cryo-EM Structure of MK-6240 Bound to Tau Paired Helical Filaments. (Kunach P, et al., 2024)
Select Service
Technical Advancements in Cryo-EM
A. High-Resolution Imaging Techniques
1. Single Particle Cryo-EM
Single-Particle Cryo-EM is an important branch of cryo-EM technology, which reconstructs three-dimensional structures by analyzing a large number of two-dimensional projection images. In recent years, this technology has made significant progress in improving resolution:
- Resolution breakthrough: Single-particle cryo-EM has been able to achieve near-atomic resolution (< 3 Å), and even 2 Å or lower in some cases. This is due to the use of direct electron detectors, the optimization of image processing algorithms, and the improvement of sample preparation technology.
- Technical tools and algorithms: Advanced software tools such as RELION-3 and RELION-4, as well as the CTFFIND4 algorithm, have greatly improved data processing efficiency and resolution. In addition, technologies such as electron counting beam induced motion correction are also used to further improve resolution.
- Application range: Single-particle cryo-EM is widely used in the study of membrane proteins, macromolecular complexes, and dynamic biological processes, and is particularly suitable for the analysis of complex samples.
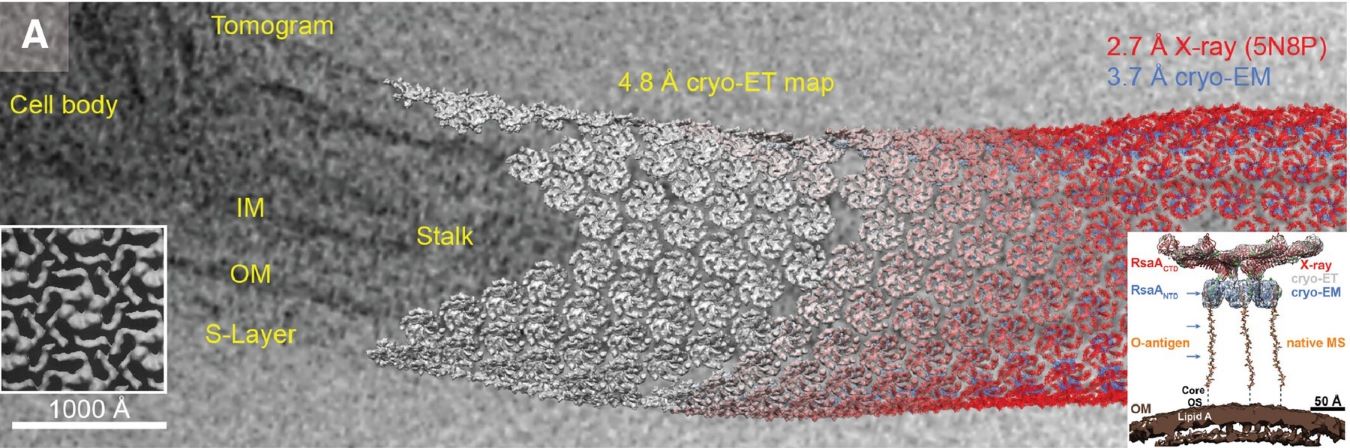
2. Cryo-Electron Tomography
Cryo-Electron Tomography (Cryo-ET) is a 3D imaging technique that reconstructs a 3D structure by rotating the sample and acquiring 2D images at different angles. Its advantage is that it can provide higher resolution than traditional 2D imaging:
- Resolution level: Cryo-ET can achieve a resolution of 2-5 Å, and even atomic resolution in some cases.
- Technical advantages: Combining the high resolution of transmission electron microscopy and the advantages of 3D reconstruction technology, this method can observe the 3D structure of biological macromolecules in a near physiological state.
- Application areas: Cryo-ET is widely used to study the 3D morphology of organelles, virus particles and other complex cell structures.
B. Data Processing and Analysis
1. Cross-Modal Alignment
Cross-modal alignment is an important step in Cryo-EM data processing, which aims to combine cryo-EM images with protein sequences or other bioinformatics data to improve the accuracy of structural analysis.
2. Deep Learning in Cryo-EM
- Automated image processing: Deep learning models significantly reduce the need for manual operations, such as particle picking, denoising, and image enhancement. For example, convolutional neural network-based particle picking pipelines and self-supervised denoising models (such as DNN denoising) have greatly improved data processing efficiency.
- Structural modeling: Deep learning models such as AlphaFold and DeepTracer are used to predict protein three-dimensional structures from Cryo-EM density maps. These models can generate high-resolution structures close to the native state by integrating multiple complementary data (such as protein sequences, template structures, etc.).
- Quality assessment: Deep learning-based quality scoring tools (such as DAQ-Score) can detect map-model compatibility outliers, thereby improving the accuracy of structural analysis.
- Post-processing optimization: Deep learning is also used in the post-processing of Cryo-EM data, such as DeepEMhancer, which improves image contrast and resolution through self-supervised learning.
C. Overcoming Preferred Orientation
1. Self-Supervised Deep Learning
Self-supervised deep learning methods have recently advanced in addressing the preferred orientation problem. For instance, spISONet, an end-to-end approach, uses unsampled view information to achieve high-quality 3D reconstructions. By incorporating atlas anisotropy and particle misalignment correction modules, it enhances angular isotropy and particle alignment accuracy, improving 3D reconstruction quality. Verified on datasets like β-galactosidase, HA trimer, and HIV VLP, spISONet has demonstrated its potential for enhanced image quality and reconstruction accuracy.
These methods eliminate additional sample preparation, directly optimize orientation issues from existing data, reduce experimental costs, improve processing efficiency, and avoid complexities and biases of traditional approaches.
2. Tilting Techniques
Tilt technology is another important method to solve the problem of preferred orientation. By tilting the sample stage during data acquisition, the orientation diversity of the sample can be increased, thereby reducing reconstruction distortion caused by orientation bias. However, tilt technology also has some limitations. For example, the image quality may be reduced due to excessive tilt angles, and the increase in ice thickness may also limit the effectiveness of tilting.
Future Directions and Potential
A. Emerging Trends in Cryo-EM
1. Multi-Particle Refinement
Multi-particle refinement is an important development direction in cryo-electron microscopy research. By combining multiple single-particle images, the three-dimensional structure of biological macromolecular complexes can be analyzed. This method can overcome the problems of heterogeneity and dynamic changes that may exist in the sample preparation process, thereby improving the accuracy of the analysis. For example, using multi-particle refinement technology, researchers successfully resolved the structure of the ribosome-antibiotic complex with a resolution of 3.5 Å. In addition, multi-particle refinement has also been used in the field of subtomogram averaging, further improving the quality of three-dimensional reconstruction.
2. In-Cell Cryo-EM
In-Cell Cryo-EM allows scientists to study the structure and function of biomolecules under near-physiological conditions. This technique avoids the bias caused by sample purification and crystallization in traditional cryo-EM by preparing and imaging samples directly in cells. For example, in-cell cryo-EM has been used to study the interaction between viral coat proteins and antibodies, and in cancer research, it has revealed the behavior of cancer cells and their interaction with therapeutic agents. Further development of this technology is expected to promote a deeper understanding of complex biological systems.
3. Time-resolved Cryo-EM
Time-resolved Cryo-EM technology can capture the dynamic process of biomolecules on a millisecond time scale. For example, through rapid freezing technology, researchers can study the conformational changes of protein complexes on a millisecond time scale. The application of this technology will greatly promote the understanding of the functional mechanism of biomolecules.
B. Integration with Other Techniques
Cryo-EM is gradually being combined with other imaging techniques (such as super-resolution microscopy, X-ray crystallography, NMR spectroscopy, mass spectrometry, etc.) to form a multimodal research platform. For example, the method of combining super-resolution microscopy and cryo-EM can significantly improve the quality of cell structure analysis. In addition, the combination of cryo-EM and drug development also shows great potential, especially in the structural analysis and functional verification of new drug targets.
Select Service
Cryo-EM continues to transform the landscape of structural biology research, demonstrating unparalleled capabilities from viral structure determination to neurodegenerative disease studies. As a professional cryo-EM service provider, our state-of-the-art facilities and experienced team are ready to support your research projects. Whether you're conducting viral research, protein structure analysis, or drug development, we offer customized solutions tailored to your specific needs. Contact us to discuss how we can advance your research through our comprehensive cryo-EM services.
References
- Nogales E. The development of cryo-EM into a mainstream structural biology technique. Nature Methods. 2016, 13(1): 24-27.
- Sirohi D, Chen Z, Sun L, et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016, 352(6284): 467-470.
- von Loeffelholz O, Natchiar S K, Djabeur N, et al. Focused classification and refinement in high-resolution cryo-EM structural analysis of ribosome complexes. Current Opinion in Structural Biology. 2017, 46: 140-148.
- Callaway E. Revolutionary cryo-EM is taking over structural biology. Nature. 2020, 578(7794): 201-202.
- Wrapp D, Wang N, Corbett K S, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020, 367(6483): 1260-1263.
- Cushing V I, Koh A F, Feng J, et al. High-resolution cryo-EM of the human CDK-activating kinase for structure-based drug design. Nature Communications. 2024, 15(1): 2265.
- Haack D B, Rudolfs B, Jin S, et al. Scaffold-enabled high-resolution cryo-EM structure determination of RNA. bioRxiv. 2024: 2024.06. 10.598011.
- Kunach P, Vaquer-Alicea J, Smith M S, et al. Cryo-EM structure of Alzheimer's disease tau filaments with PET ligand MK-6240. Nature Communications. 2024, 15(1): 8497.
- Liu Y T, Fan H, Hu J J, et al. Overcoming the preferred orientation problem in cryoEM with self-supervised deep-learning. bioRxiv, 2024.

-1.jpg)