Cryo-EM for Small Proteins
Cryo-electron microscopy (cryo-EM) is a form of transmission electron microscopy (TEM) where biological samples are studied at cryogenic temperatures. It is becoming a more and more important technique in the field of structural biology, as has been manifested by many once hard-to-determine macromolecular structures. With the rapid advancement of both hardware and software, the resolution of cryo-EM maps is improving steadily. In some cases, near-atomic resolution had been obtained, including those of viruses, ribosomes, mitochondria, ion channels, and enzyme complexes as small as 150 kD.
However, very small proteins remain challenging to study using EM because of the difficulty in their visualization, alignment and validation. To address this technical challenge, Creative Biostructure is devoted to optimizing our EM protocol and making use of available resources to provide high-resolution imaging and three-dimensional reconstruction services for a broad spectrum of small proteins of interest. Our strategies include:
- Improved sample preparation with ultra-thin vitreous ice layer
- Selection of binding agents such as Fabs
- Optimization of the electron dose
- Variation of defocus
- Energy filter and correction of beam-induced motion
- Use of phase plate for contrast enhancement
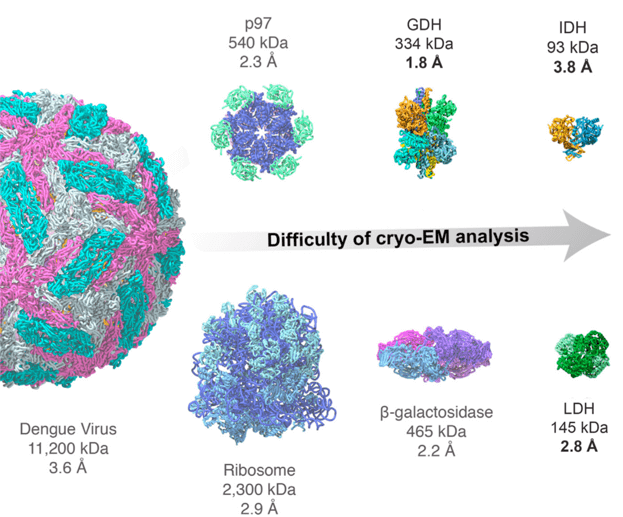 Figure 1. Cryo-EM for small proteins
Figure 1. Cryo-EM for small proteins
The advantages of using our EM platform for the structure determination of proteins with small molecular weight include sample-saving, fast turnaround time, and cost-effective. Creative Biostructure promises to work closely with our customers to provide tailored cryo-EM strategies for determining the structure of biologically or structurally important small proteins, with a resolution comparable to traditional X-ray crystallography techniques.
Please feel free to contact us for a detailed quote.
Ordering Process
References:
- Merk A, et al. (2016) “Breaking cryo-EM resolution barriers to facilitate drug discovery”. Cell 165(7):1698-1707.
- Wu S, et al. (2012) “Fabs enable single particle cryo-EM studies of small proteins”. Structure 20(4):582-592.
