Determination of Disulfide Bond Connectivities in Pharmaceutical Peptides
The covalent bond formed between two cysteine sulfur atoms is called a disulfide bond, which plays a decisive role in the functionality, correct folding and stability of the protein. At the same time, disulfide bonds also appear in secretory proteins or cell surface proteins to maintain stability.
Peptides rich in disulfide bonds are very common in plants and venoms, and they will endow a series of peptides with special structures and functions. With the increasing demand for drug development, people have a strong interest in characterizing new peptides rich in disulfide bonds. At present, disulfide-rich peptides have been developed for the treatment of diabetes, pain and other diseases and diseases.
NMR spectroscopy can be a very convenient and powerful tool for determining the connectivity of disulfides, and as a non-destructive technology, other analyses can be carried out later. In many cases, nuclear magnetic resonance spectroscopy is the preferred technique for determining the structure of disulfide-rich peptides.
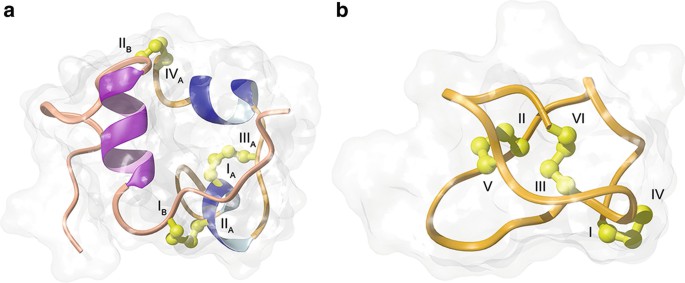 Figure 1. Three-dimensional structures of insulin (a) and ziconotide (b). (Wilson & Daly, 2018)
Figure 1. Three-dimensional structures of insulin (a) and ziconotide (b). (Wilson & Daly, 2018)
Our Service
As an expert in the field of nuclear magnetic resonance, Creative Biostructure uses NMR to provide customers with analytical services to clarify the disulfide bond connection in drug peptides. Disulfide bond connectivity is a key step in determining the structure-function relationship, which helps to develop more effective and selective analogues.
Our NMR-based Approaches
- Simple comparative analysis of chemical shifts of analogues of peptides with good characteristics.
- Using high-resolution 3D structure to determine disulfide bond connectivity.
- Measurement of scalar couplings across diselenide bonds.
- The use of computational Bayesian methodologies.
Optional Analysis Schemes
| Schemes | Scope of use |
|---|---|
| Chemical shift analysis | For peptides with clear structural characteristics, chemical shift analysis without structural calculation can provide a simple and convenient method to determine whether mutations have an impact on the formation of natural disulfide bonds. |
| High-resolution 3D structure | In the absence of suitable peptide structures for comparison, a high-resolution structure is needed to determine the disulfide bond connectivity of the new peptide. |
| Measurement of spin coupling on disulfide bonds | The only way to determine the disulfide bond connectivity of peptides directly by NMR is to observe the coupling between disulfide bonds. Substitution of cysteine residues with selenocysteine is a direct method to measure the coupling between links, which can measure the scalar coupling between the nucleus in Cys residues on one side of the disulfide bond and the nucleus in the covalently linked Cys residues. |
| Computational Methodology | A method for inferring the connectivity of disulfide bonds directly from the topological structure of cysteine residues. |
Applications of Disulfide Bond Determination
- Characterization of new disulfide-rich peptides.
- Determine whether the mutation influences the formation of the natural disulfide bond.
- Test whether the peptide is folded correctly.
- Confirm the correct disulfide bond connection in the peptide therapeutic agent.
Distance information between cysteines.
Advantages of NMR in the Determination of Disulfide Bond Connectivities
When there are multiple disulfide bonds and they gather at the core of the molecule, it may be difficult to determine the disulfide bond connectivity of the peptide. NMR spectroscopy provides a series of methods for analyzing disulfide bonds. Importantly, since this technology is non-destructive, additional analysis can be performed after the NMR spectroscopy study.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- Wilson D, Daly N L. Approaches to delineate disulfide connectivities in pharmaceutical peptides[M]//Modern Magnetic Resonance. Springer International Publishing. 2018, 3: 2021-2034.
