Hydration and Molecular Dynamics of Collagen
Collagen is the most abundant protein in the human body and plays a key role in the biological functions of various hard and soft tissues. With its unique amino acid composition and three-dimensional structure, collagen gives tensile strength to bones, ligaments, tendons, cartilage and other tissues. Solid-state nuclear magnetic resonance spectroscopy is a very suitable method for studying complete biological tissues.
As an expert in the field of nuclear magnetic resonance, Creative Biostructure relies on solid-state NMR technology to provide our customers with analysis services on the structure, dynamics, and hydration of collagen in various biological tissues.
Why Use Solid-state NMR for Collagen Analysis
- Solid-state NMR study of collagen is helpful to clarify the structure and dynamics of a protein in biological tissues and the specificity of hydration of collagen in hard and soft tissues.
- The advantage of solid-state nuclear magnetic resonance spectroscopy is that it can study biological tissues, because it comes from natural sources, without any preliminary sample processing.
- The individual (macromolecular) components of the tissue can be spectroscopically separated using appropriate NMR techniques.
- Solid-state NMR can be applied to complete bone samples to study the structure and kinetics of the tissue, solid-state NMR, collagen hydration, and collagen kinetics.
- About 50% of the protein in cartilage is type II collagen. Complete cartilage samples can be studied by solid-state magnetic resonance technology.
Analysis Services of Collagen Hydration and Dynamics in Cartilage
Sample Processing and Sample Requirements
Place approximately 50mg of cartilage sample into a 4 mm MAS rotor with PTFE insert and seal. Deuterified water (D2O) can be used to exchange some water in cartilage to lock in 2H frequency to obtain better field stability during the experiment.
Our Services
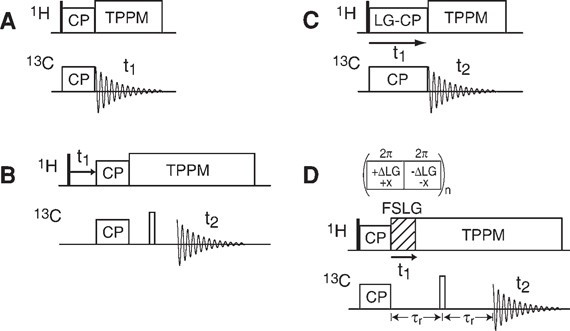 Figure 1. Pulse sequences for the CP MAS experiment (A), the WISE experiment (B), the LG-CP experiment (C), and the DIPSHIFT experiment (D) (Huster et al., 2004)
Figure 1. Pulse sequences for the CP MAS experiment (A), the WISE experiment (B), the LG-CP experiment (C), and the DIPSHIFT experiment (D) (Huster et al., 2004)
| Optional Experiments | Description |
|---|---|
| CP (cross-polarization) MAS experiment | CP experiment is the basic component of almost all solid-state NMR experiments. The CP MAS spectrum of cartilage is mainly composed of the resonance of rigid collagen, while the signal from glycosaminoglycan is only observed at low intensity. |
| Wideline separation (WISE) experiment | It is the measurement of 1H-1H wide lines. It can be used to study the molecular dynamics of collagen in natural cartilage tissue. To obtain qualitative liquidity information from this simple experiment: the narrower the line, the greater the mobility of molecular sites. |
| Lee-Goldburg CP (LG-CP) experiment | It is the measurement of 1H-13C dipolar couplings by Lee-Goldburg CP. It can be used to conduct more quantitative research on the segmental movement of cartilage collagen. |
| Dipolar-Chemical Shift Correlation (DIPSHIFT) experiment | It is the measurement of 1H-13C dipolar couplings by DIPSHIFT. Using the DipShift experiment, the molecular dynamics of collagen in the fully hydrated cartilage tissue of pig joints can be measured. |
Analysis Services of Collagen Hydration and Dynamics in Bone
Most of the work is devoted to the study of the structure, kinetics, and hydration of bone collagen. In addition, solid-state magnetic resonance technology is a very suitable tool for analyzing problems and supporting bone tissue engineering research in regenerative medicine. Common NMR technologies include:
- 13C CPMAS NMR
- 13C-31P REDOR
- Separated local field experiments
Applications of Collagen Analysis in Bone
- Helps identify other bone components.
- Analyze the mechanical properties and bone health of bones.
- Analyze the role of water in bone and bone hydration.
- Evaluate bone aging.
- Analysis of bone tissue engineering in vivo.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- Huster D, Schiller J, Arnold K. Dynamics of collagen in articular cartilage studied by solid-state NMR methods. Cartilage and Osteoarthritis: Volume 2: Structure and In Vivo Analysis. 2004: 303-318.
