Silk Materials Analysis
Silk, as a potential biomedical material and textile material, has received widespread attention. Understanding the structure and dynamic characteristics of natural silk materials is crucial for developing these applications of silk. The most detailed picture of silk structure and kinetics can be obtained at the molecular level by using solid and solution NMR.
As an expert in the field of nuclear magnetic resonance, Creative Biostructure relies on solution NMR and solid NMR technology to provide customers with analysis services for the structure of multi silk protein solution and silk biomaterials such as hydrogels, powders, films, fibers and sponges.
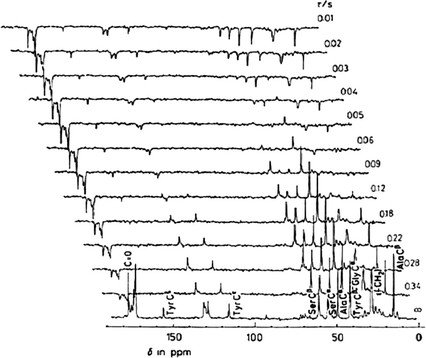 Figure 1. Partially relaxed 13C NMR spectra of middle silk gland portion of living B. mori mature larva at room temperature. (Asakura & Tasei, 2017)
Figure 1. Partially relaxed 13C NMR spectra of middle silk gland portion of living B. mori mature larva at room temperature. (Asakura & Tasei, 2017)
Our Methods
Solution NMR
We can use solution NMR to obtain the solution structure of silk fibroin SF (the main component of silk protein), including the liquid silk fibroin (SF) stored in the living silk gland. Or use mature multi-dimensional NMR technology and isotope labeling technology to clarify the atomic structure. In addition, we can also use solution NMR to study the solution structure and dynamics of the interaction between water molecules and silk materials.
Solid-state NMR
We can use ssNMR to analyze the structure and dynamics of several forms of silk materials for customers, including hydrogels, powders, films, fibers and sponges, which are very useful in the development of silk to biological materials.
Analysis of the Dynamics of Silk Fibroin Stored in Living Silkworm
The shape and size of the mature silkworm larvae are suitable for insertion into the 13C solution NMR sample tube. Therefore, we can directly observe SF stored in live silkworms by 13C NMR without extracting SF from silkworms. Since the liquid SF extracted from the silk gland is easy to be affected by external forces such as shear stress and changes its structure during extraction or drying, NMR observation in vivo is very effective.
Analysis of the Solution Structure of Silk Fibroin at Atomic Level
We can determine the solution structure of SF according to the chemical shifts of 1H, 13C and 15N solutions.
The 1H, 13C and 15N nuclei of SF extracted from silk gland were sequentially allocated through bond correlation experiment, 2D double resonance and 3D double resonance and triple resonance.
The twist angle constraint of the main chain is derived from the database analysis of 13Cα, 13Cβ, 13CO, 1Hα, 1HN and 15N chemical shifts using TALOS-N.
Analysis of the Dynamics of Water Molecules Interacted with Silk Fibroin
We can use NMR relaxation to monitor the dynamics and distribution of water molecules interacting with SF. The kinetics of unfrozen bound water molecules absorbed in SF fibers, films and powders was analyzed by 1H pulsed NMR.
Analysis of the Dynamics of Hydrated Silk Cocoon, Sericin, and Fibroin
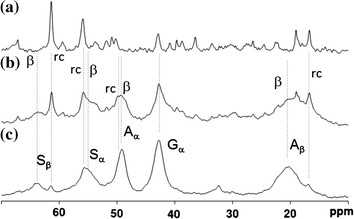 Figure 2. a 13C r-INEPT, b 13C DD/MAS and c 13C CP/MAS NMR spectra of natural silk cocoon fiber in the hydrated state. (Asakura & Tasei, 2017)
Figure 2. a 13C r-INEPT, b 13C DD/MAS and c 13C CP/MAS NMR spectra of natural silk cocoon fiber in the hydrated state. (Asakura & Tasei, 2017)
13C CP / MAS NMR can be used to analyze the structure and dynamics of rigid components.
13C refocusing INEPT NMR can be used to obtain the only soft and mobile component in the material.
13C DD / MAS NMR can determine the fraction of mobile and fixed components.
The above three methods can provide different perspectives for the dynamic behavior of hydrated silk samples and can be used together to characterize their local structure and conformation.
Analysis of the Fraction of Several Conformations of Silk Fibroin
Through deconvolution analysis of 13C DD / MAS NMR at the amino acid level based on the conformation-related 13C NMR chemical shift, we can determine the fractions of several conformations of SF in dry and hydrated state.
Analysis of the Domain of Silk Fibroin
We can obtain the distance information through 2D 13C -13C correlation spectrum with dipole-assisted rotational resonance (DARR). The complete spin connectivity within a given amino acid residue can be obtained from these spectra, which greatly contributes to spectral allocation. In addition to the strong intramolecular correlation of a given amino acid, the 2D 13C -13C correlation spectrum also shows the intermolecular interaction between residues.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- Asakura, T., & Tasei, Y. NMR studies on silk materials. Experimental Approaches of NMR Spectroscopy. 2017, 297-312.
