Cryo-EM for Antigen-Antibody Complexes
Creative Biostructure enables to advance 3D cryo-electron microscopy (cryo-EM) into antigen-antibody complex applications for drug discovery. 3D cryo-EM enables the structure determination of large protein complexes like antigen-antibody complexes, or membrane proteins. We expertise in protein production and proprietary expression and cell culture tools, including antigen-antibody complexes, make proteins of highest quality available. Your target of choice can be produced at Creative Biostructure and subsequently analyzed with cryo-EM for 3D reconstruction or epitope mapping applications.
3D cryo-EM – an emerging structure determination technique that significantly improves our understanding of biological systems. It complements existing atomic-resolution approaches like X-ray crystallography by providing in-situ context within large complexes as well as access to structures of difficult to crystallize multi-domain proteins, antibodies or integral membrane proteins. 3D Cryo-EM perfectly matches the technologies already established at CRELUX and can be easily integrated in our workflow.
Typical workflow comprises:
- Generation of high quality antigen-antibody complex of the target of interest (performed by Creative Biostructure or provided by the client).
- Cryogenic sample preparation: Small amounts are prepared and flash-frozen to form an amorphous ice phase that preserves native, solution-like conditions.
- Electron microscope imaging: An advanced transmission electron microscope is used to take high resolution 2D images of the vitrified sample in a low-exposure mode.
- Computational 3D reconstruction: Iterative computational procedures are used to aggregate the 2D images into a 3D structure. Several protocols like electron tomography, single particle analysis, or sub-volume averaging are used.
- Analysis and interpretation: The 3D models are verified and analyzed. Technological advances are making atomic resolution of large proteins possible.
The workflow designed by our experts includes an analysis process and a sample optimization system that automatically determines the buffer, pH, and ligand conditions necessary to maintain optimal stability of the sample during analysis. This represents a critical step prior to the subsequent EM analysis, as it ensures its overall success. EM-based structural analysis has emerged as an important complementary technique to traditional methods, such as X-ray crystallography and nuclear magnetic resonance (NMR).
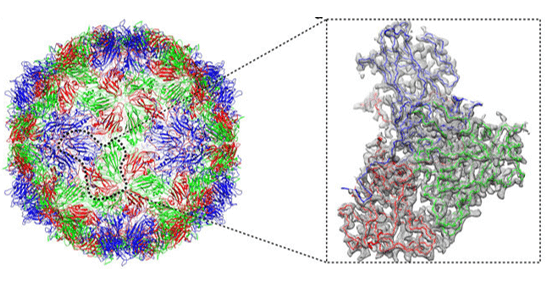 Figure 1. Atomic structures of Coxsackievirus A6 and its complex with a neutralizing antibody
Figure 1. Atomic structures of Coxsackievirus A6 and its complex with a neutralizing antibody
There is a growing demand for structural studies from the pharmaceutical industry, driven by the need for a deeper scientific understanding, and also because of stronger regulatory constraints. In the field of biologics, EM analysis has proven to be capable of delivering critical information for antibody selection, epitope mapping and formulation.
Please feel free to contact us for a detailed quote.
Ordering Process
References
- Longfa Xu, et al. Atomic structures of Coxsackievirus A6 and its complex with a neutralizing antibody. Nature Communications 8, Article number: 505(2017).
- Mayuri Tarasuk, et al. Human single-chain variable fragment antibody inhibits macrophage migration inhibitory factor tautomerase activity. International Journal of Molecular Medicine. January 13, 2014.

