Natural Product Analysis
Structural identification of natural products is a very important and active research field, from which important bioactive components can be found and utilized, which is the key to the current research and development of new drugs. Nuclear magnetic resonance (NMR) spectroscopy has developed into an indispensable tool for the structural determination of new compounds and the chemical analysis of natural products. From the characterization of synthetic products to the study of molecular structures of biological systems, this technology has a wide range of applications. This method is usually used to study the structure and conformation and to analyze the molecular interaction and motion.
As an expert in the field of nuclear magnetic resonance, Creative Biostructure provides our customers with quantitative NMR analysis of natural products, skeleton structure analysis, stereochemistry, and three-dimensional structure analysis services.
Natural Product Quantification Service
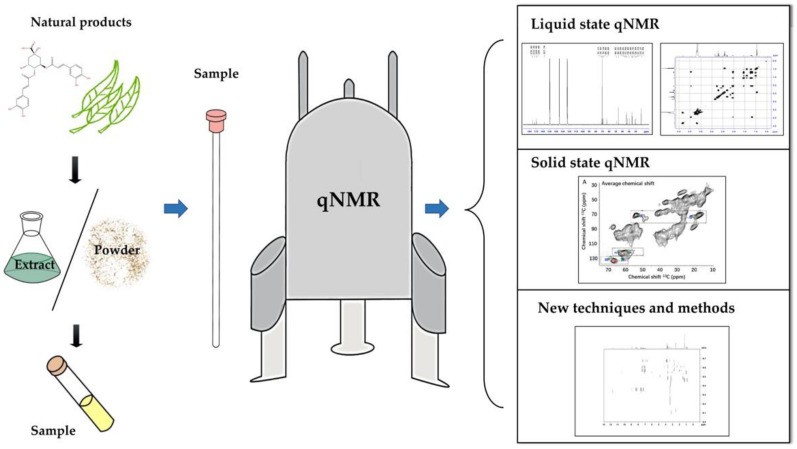 Figure 1. Quantification of natural products (Wang et al., 2021)
Figure 1. Quantification of natural products (Wang et al., 2021)
Due to the special composition of natural products, there are few corresponding reference substances, which increases the cost of quantitative detection of many natural products. When the impurity peak does not interfere with the sample peak or the impurity has no peak, the quantitative NMR does not care about the sample impurity, does not need the standard reference material, and the whole process will not cause damage to the sample, which makes it very suitable for the quantitative detection of natural products.
Our quantitative NMR services cover a variety of atomic nuclei, such as 1H, 13C, 19F, 17O, 31P, etc. Among them, 1H NMR is the most widely used because of its higher sensitivity and satisfactory quantitative relationship. At present, the minimum error range can be controlled within 2%.
Determination Service of Skeletal Structures of Natural Products
Through NMR, we can not only provide customers with basic information about the plane structure of natural products but also provide their configuration and conformation. Specifically, we characterized the molecular skeleton of natural products through the correlation experiments of 1H-1H and 1H-13C through direct bonds such as COSY and HMBC. The 1H-1H correlation spectrum is combined with the single-bond 13C-1H correlation spectrum to determine the fragments of protonated carbon, and then the long-range 13C-1H correlation is used to link the fragments. Our approach also includes:
- TOCSY spectra
- One-bond 1H-13C (and 1H-15N) spectra
- N-bond (n = 2 or 3) 1H-13C shift correlation spectra
Stereochemical Determination and Conformational Analysis
By evaluating 1H-1H spin coupling constant (3JHH), chemical shift, and nuclear Overhauser effect (NOE), we can determine the relative stereochemistry of ring compounds with rigid three-membered to six-membered rings.
In contrast, the relative configuration distribution of multi-substituted acyclic chains and macrocycles is more difficult, because the geometric flexibility of this system makes the NOE strength less reliable for stereochemical distribution. To solve these problems in the relative configuration allocation of organic molecules, our strategies for customers include:
- J-based configuration analysis (JBCA)
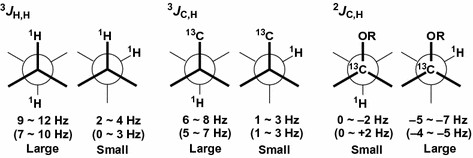 Figure 2. Empirical and qualitative Karplus-type dihedral angle dependence of coupling constants and their classification into large and small according to the magnitude of the absolute values (Matsumori & Murata, 2017)
Figure 2. Empirical and qualitative Karplus-type dihedral angle dependence of coupling constants and their classification into large and small according to the magnitude of the absolute values (Matsumori & Murata, 2017)
This method not only uses the experience of 3JHH and qualitative Karplus-type dihedral angle dependence, but also uses long-range C-H coupling constants (2,3JC, H) value to determine the relative stereochemistry of two adjacent or alternate stereocenters. Usually, it is often observed in the multi-substituted acyclic chain of natural products.
In the JBCA method, it is assumed that the C-C bond can be represented by three staggered rotational isomers (one anti-rotational isomer and two rotational isomers), and the coupling constant is qualitatively classified as small and large) For acyclic carbon chains with small-volume substituents (such as hydroxyl and methyl), this assumption is usually reasonable, and they will hardly lead to a significant deviation from the staggered position.
- Universal NMR database (UDB)
Used to specify the stereochemistry of (acyclic) compounds. A highly functional molecule can be considered as the sum of independent stereo-central clusters (stereo-clusters). The stereochemistry of each independent stereo cluster can be determined by comparing the model compound library with the natural products unknown to stereochemistry by NMR data.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
References
- Wang Z F, You Y L, Li F F, et al. Research progress of NMR in natural product quantification. Molecules. 2021, 26(20): 6308.
- Matsumori N, Murata M. NMR studies on natural product—stereochemical determination and conformational analysis in solution and in membrane. Experimental Approaches of NMR Spectroscopy: Methodology and Application to Life Science and Materials Science. 2018: 383-414.
