E3-Ligases
Ubiquitin ligase, also known as E3 ubiquitin ligase, is an enzyme that transfers ubiquitin molecules to a lysine of a target protein. It first recruits an E2 ubiquitin-conjugating enzyme loaded with ubiquitin, then recognizes a target protein, and assists or directly catalyzes the ubiquitin transfer from the E2 enzyme to the target protein.
Polyubiquitin-modified target proteins (such as denatured, misfolded or overexpressed proteins) can be degraded through the ubiquitination pathway. Ubiquitination modification plays an important role in cell cycle regulation, endocytosis, signal transduction, DNA repair, protein quality control, apoptosis, and other processes. E3 ligase also plays a crucial regulatory role in oncology and immunology. It is a novel drug discovery target that hasn't been fully studied yet. Cryogenic electron microscopy (cryo-EM) can use specific chemical probes to visualize the process of ubiquitination, thus revealing the essential function of E3 ligase.
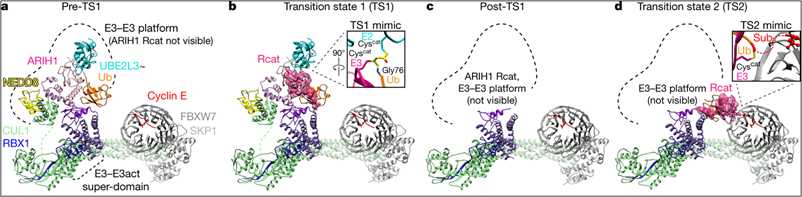 Figure 1. A snapshot of intermediates during ubiquitination (Horn-Ghetko D, et al. 2021)
Figure 1. A snapshot of intermediates during ubiquitination (Horn-Ghetko D, et al. 2021)
At present, cryo-EM is increasingly used in drug discovery, which can visualize the pathogenesis of cancer or tumors. It has solved the structures of E3 ligase and other macromolecules, which makes structure-based drug discovery possible.
Our cryo-EM platform shows advantages in studying the structural and conformational changes of E3 ligases. If you are interested in our cryo-EM services for drug discovery, please feel free to contact us. We are looking forward to cooperating with you.
Ordering Process
Reference
- Horn-Ghetko D, et al. Ubiquitin ligation to F-box protein targets by SCF–RBR E3–E3 super-assembly. Nature. 2021. 590(7847): 671-676.
