NMR Analysis for Biopharmaceutical Development
Nuclear Magnetic Resonance (NMR) is a crucial method in biochemistry, structural biology and biopharmaceuticals. It can be used not only to study the structure and dynamics of biological macromolecules, but also to study biomacromolecule-ligand interactions as NMR technology is compatible with physiological conditions and gives precise information of the structure and dynamics of protein-drug molecular complexes.
Creative Biostructure is committed to NMR analysis with premium quality. Our state-of-the-art NMR platform combines advanced instruments and the latest software to deliver high resolution spectral data, which helps elucidate the structure of some of the most sophisticated molecules as well as their interactions. After being labeled with isotopes such as 1H, 13C, 15N and 31P, biomacromolecules can be used in spectral processing with enhanced sensitivity to support biopharmaceutical development.
Why Creative Biostructure?
- Various isotope-labeling strategies for NMR studies.
- NMR analysis provides the detail information of the molecular structures and dynamics of protein-ligand interactions.
- Combine NMR with Liquid Chromatography-Mass Spectroscopy (LC-MS) to identify the pharmaceutical substances and impurities.
- State-of-the-art NMR instrumentation and powerful computer programs to ensure products meet quality specifications.
- A team of experts in pharmaceutical development, clinical safety and efficacy evaluation.
- High Resolution NMR analysis services in compliance with GLP and GMP to protect your research.
- Our services cover the whole process of pharmaceutical development from early state research to post-approval studies.
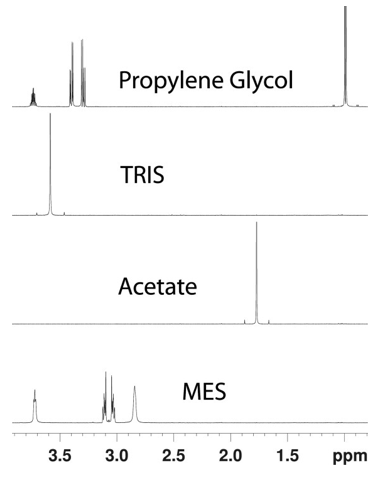 Figure 1. 1H NMR spectra of various analytes of interest.
Figure 1. 1H NMR spectra of various analytes of interest.
Creative Biostructure promises to work closely with our customers to provide excellent services of pharmaceutical development with unique biological insights.
Please feel free to contact us for a detailed quote.
Ordering Process
References
- Pawanpreet Singh, Renu Chadha, A new polymorph of ciprofloxacin saccharinate: Structural characterization and pharmaceutical profile. J Pharm Biomed Anal, 2017; 146:7-14.
- Ken Skidmore, Daniel Hewitt, and Yung-Hsiang Kao, Quantitation and Characterization of Process Impurities and Extractables in Protein-Containing Solutions Using Proton NMR as a General Tool. Biotechnol Prog, 2012; 28:1526-1533.

