Analysis of Soluble Protein Complexes
Solid-state NMR (SS-NMR) of proteins requires that these molecules be immobilized, usually by crystallization, freezing, or lyophilization. In recent years, MAS solid-state NMR has emerged as a technique for studying soluble protein complexes. It was found that high molecular weight complexes do not require crystallization to obtain immobilized samples for solid-state NMR studies.
As an expert in the field of nuclear magnetic resonance, Creative Biostructure can provide customers with analysis services of soluble protein complexes by Sedimentation Solute NMR (SedNMR) technology. In addition, we offer FROSTY*/ sedNMR technical services, by which inhomogeneous line broadening due to chemical shift disorder and anisotropic magnetic susceptibility present in solid samples can be minimized.
*Freezing Rotational diffusion Of protein Solutions at low Temperature and high viscositY
Our Technology 1. Sedimented Solute NMR (SedNMR)
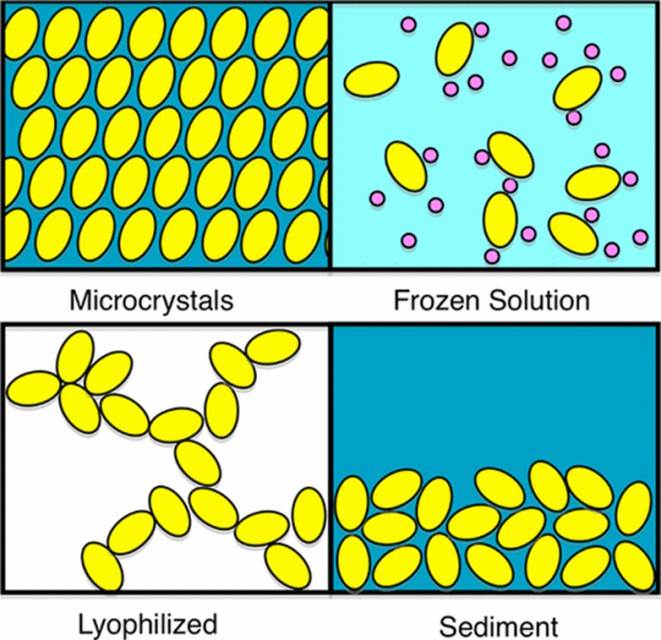 Figure 1. Different techniques of sample preparation for SS-NMR experiments (Bertini et al., 2013)
Figure 1. Different techniques of sample preparation for SS-NMR experiments (Bertini et al., 2013)
SS-NMR of proteins requires that these molecules be immobilized. However, self-crowding can also slow molecular rotation sufficiently to prevent nuclear interaction averaging. Precipitated solute NMR (SedNMR) provides a simple method to prepare biological samples for SS-NMR experiments with minimal perturbation.
Our Technology 2. FROSTY / sedNMR
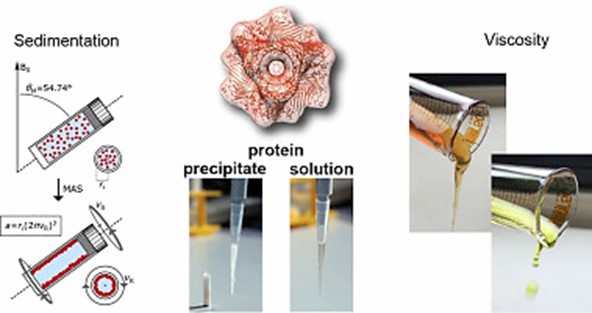 Figure 2. FROSTY / sedNMR (Sarkar et al., 2016)
Figure 2. FROSTY / sedNMR (Sarkar et al., 2016)
Sedimentation induced by sample rotation impairs rotational diffusion of proteins and enables cross-polarization transfer based on dipolar coupling. Additionally, viscosity aids in the immobilization of molecules in the sample. Natural deep eutectic solvents (NADES) have very high viscosity and can replace water in living organisms. The FROSTY/sedNMR method avoids sample preparation problems. It is no longer necessary to select suitable precipitation conditions. Inhomogeneous line broadening due to chemical shift disorder and anisotropic magnetic susceptibility present in solid samples can be minimized by this method.
How We Obtain High Resolution Spectra in FROSTY/sedNMR
- To generate high-resolution proton spectra in the solid state, we can chemically suppress the 1H-1H dipole coupling by perdeuteration of the sample.
- The corresponding fully deuterated proteins were recrystallized from a buffer containing 90% D2O, and MAS solid-state NMR spectra with ultra-high resolution 1H detection were obtained at intermediate rotation frequencies (10-20 kHz).
- Using specific protonated amino acid precursors to record high-resolution spectra of methyl protons in perdeuterated peptides and proteins.
- Recrystallized samples from buffers containing 100% H2O obtained reasonably high resolution at fast spins (60kHz).
Analysis of Soluble Protein Complexes
By using FROSTY/SedNMR method, the analysis range of soluble protein complexes includes,
- Analysis of metal binding sites in protein complexes
- Study the formation of oligomer
- Analysis of homologous oligomeric protein complexes
- Analysis of protein complexes lacking symmetry
- Characterization of molecular chaperone amyloid interaction
In addition, if the molecular weight of phospholipid nano disk reconstituted membrane protein exceeds the molecular weight limit imposed by solution NMR, the combination of heterotopic sedimentation and FROSTY/sedNMR provides an alternative method for system structure analysis.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
References
- Bertini I, et al. SedNMR: on the edge between solution and solid-state NMR. Accounts of Chemical Research. 2013. 46(9): 2059-2069.
- Sarkar R, et al. Immobilization of soluble protein complexes in MAS solid-state NMR: Sedimentation versus viscosity. Solid State Nuclear Magnetic Resonance. 2016. 76: 7-14.
