Characterization of Peptide Toxin Structure and Function
Peptide toxin is an effective probe for studying the structure and function of ion channels and receptors, which usually has high selectivity. Therefore, it is of great significance to the pharmaceutical and biotechnology industries as a treatment guide and pharmacological tool. Several venom-derived peptides have become drugs for treating hypertension, type 2 diabetes, neuropathic pain, and other diseases. NMR is particularly suitable for the study of peptide toxins. Once the peptide structure is determined, it can be used as the basis for modification to enhance or modify its activity and target selectivity, or simulate its active scaffold in the minimized peptide analog or non-peptide-based peptide analog.
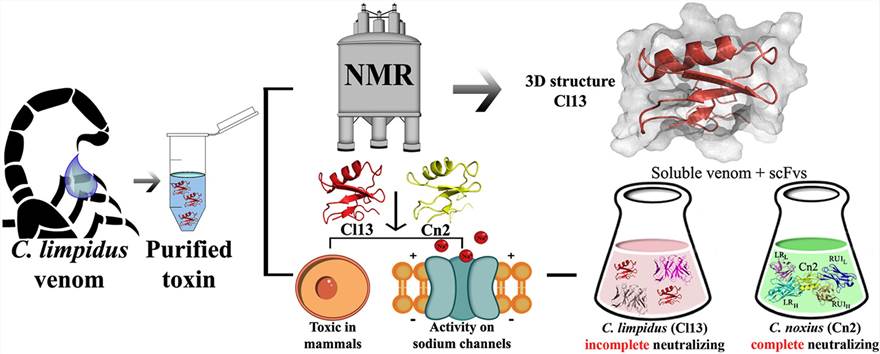 Figure 1. The three-dimensional structure of the toxic peptide. (López-Giraldo et al., 2020)
Figure 1. The three-dimensional structure of the toxic peptide. (López-Giraldo et al., 2020)
As an expert in the field of nuclear magnetic resonance, Creative Biostructure uses NMR technology to provide customers with structural analysis services of peptide toxins and helps customers explore the relationship between the structure and function of peptide toxins.
Why NMR is Suitable for Peptide Toxin Characterization
- Synthetically accessible. Allows the production of milligram quantities of sample needed for NMR analysis.
- Soluble. Enables suitable sample conditions for NMR analysis.
- Stable. Ensures no degradation of samples during NMR analysis.
- SS-rich. This leads to good dispersion of signals and simplifies spectra.
- Small. Minimizes signal overlap problems and obviates the need for isotope labeling, as is typically required for NMR of larger proteins.
Our Working Process
Producing peptides for NMR studies.
Assessing folding.
Resonance assignments.
Defining disulfide pairings.
Determining three-dimensional structures.
Interpreting the structures.
Sample Requirements
Samples need to be labeled with stable NMR active isotopes (such as 13C and 15N).
The prerequisite for peptide structure determination is a pure, soluble and stable sample. It is important to quantitatively evaluate the purity of peptide by liquid chromatography (it is better to confirm that its composition is consistent with the expected value by mass spectrometry) because NMR is very sensitive to the presence of impurities and secondary conformation.
Resonance Assignments
Once the solution conditions supporting the functional state of peptides are found, we can perform sequence-specific resonance assignments. If the peptide is not labeled with isotopes, this will follow the traditional approach of analyzing 2D homonuclear spectra, mainly:
Total correlation spectroscopy (TOCSY) identifies all resonances in a single amino acid spin system
Double quantum filter correlation spectroscopy (DQF-COSY), is used to identify the resonance of protons separated by three bonds (most importantly, HN-Hα and Hα-Hβ)
Nuclear Overhauser Enhanced Spectroscopy (NOESY) recognizes the spatial connectivity between residue i and its flanking residues in amino acid sequences, i - 1 and i+1
Determining Three-dimensional Structures
The precision and accuracy of the structure determined from the NMR data largely depend on the number of experimental limits and their distribution in the molecule. We begin by summarizing the major categories of restraints used.
- Distance restraints.
- Dihedral angle restraints.
- Disulfide restraints.
- Hydrogen bond restraints.
- Residual dipolar couplings (RDCs).
Once the complete set of available constraints is assembled, the structure can be determined. For unlabeled peptides, once a complete set of resonance distributions is available, a complete NOE list can be automatically generated from the NOESY spectrum.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- López-Giraldo, A. E, et al. The three-dimensional structure of the toxic peptide Cl13 from the scorpion Centruroides limpidus. Toxicon. 2020, 184, 158-166.
