Characterization of Antimicrobial and Host-Defense Peptides
Antibacterial peptides provide the original source of immunity and endow eukaryotic cells with resistance to bacteria, protozoa and viruses. Antimicrobial peptides (AMBP) and other host defense proteins and peptides provide a potential way to discover new anti-infective drugs. To design more effective and selective antimicrobial peptides from the perspective of pharmacology, it is necessary to better understand the molecular determinants involved in membrane interaction.
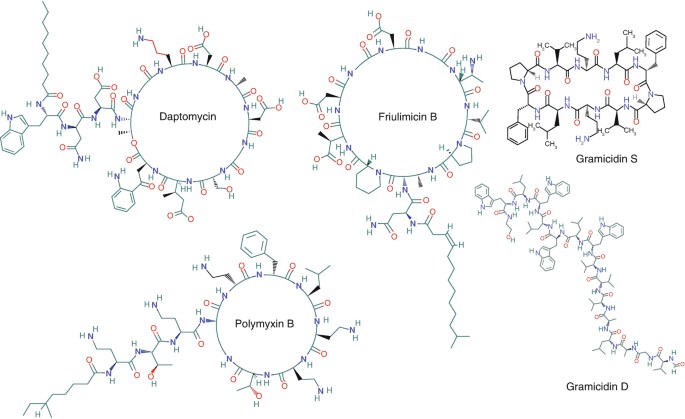 Figure 1. Structures of several nonribosomal bacterially produced antimicrobial cyclic and linear peptides as well as lipopeptides (Vogel et al., 2018)
Figure 1. Structures of several nonribosomal bacterially produced antimicrobial cyclic and linear peptides as well as lipopeptides (Vogel et al., 2018)
As an expert in the field of nuclear magnetic resonance, Creative Biostructure uses resolution solution NMR to provide customers with analysis services of the amphiphilic three-dimensional structure of antimicrobial peptides and uses solid-state NMR technology to provide customers with analysis services of the interaction between antimicrobial peptides and model lipid membranes.
Our Technology
- Proton NMR spectroscopy
In order to reduce the cost of synthesis as much as possible, it is usually carried out without adding any specific isotope markers. Therefore, the structure of these peptides can only be studied by proton nuclear magnetic resonance spectroscopy, which depends on conventional two-dimensional COSY, TOCSY and NOESY NMR experiments.
- Stable-isotope assisted NMR spectroscopy
The size of antimicrobial peptides that can be used for beneficial research only by proton NMR is limited. We used 13C and 15N isotope-labeled peptides for multi-dimensional NMR studies.
- Fluorine-19 NMR spectroscopy
A unique aspect of the Fluorine-19 NMR spectrum is that the chemical shift range of the atomic nucleus is very large. To some extent, this reflects the high sensitivity of fluorine atoms to their chemical environment.
- 31P NMR spectroscopy
Phosphorus-31 has spin ½ and 100% natural abundance and has good sensitivity, which is very useful for studying the structure and dynamics of phospholipid polar head groups. In particular, the static 31P NMR spectrum dominated by chemical shift anisotropy (CSA) can be obtained, and the magic angle rotation (MAS) technique that averages CSA to its isotropic value can be used, and finally the samples with macroscopic orientation in the magnetic field can be used. The main information obtained from the static 31P NMR spectrum is lipid polymorphism.
- 2H NMR Spectroscopy
2H NMR spectroscopy is a very powerful technology to study membrane hydrophobic nuclei by replacing acyl chain protons with deuterium. Deuterium is a nucleus with spin-1 and has a quadrupole moment. It interacts with the electric field gradient at the nucleus to produce quadrupole interaction.
Our Service
- Structure analysis of antimicrobial peptides
Peptide skeleton and amino acid side chain distribution.
3D structure of the micellar binding peptide.
Nuclear magnetic resonance spectrum analysis of host defense protein.
α-helix and β-analysis of folding formation.
- Analysis of antimicrobial peptides in membrane
Kinetic analysis of proteins and antimicrobial peptides.
Correlation analysis of side chains of aromatic and aliphatic amino acids in AMBP and antimicrobial properties.
Detect the immersion depth of peptides and proteins in the membrane.
Membrane orientation and topological structure of antimicrobial peptides.
Secondary structure analysis of antimicrobial peptides (secondary structure of 13C labeled residues, nuclear spacing, twist angle).
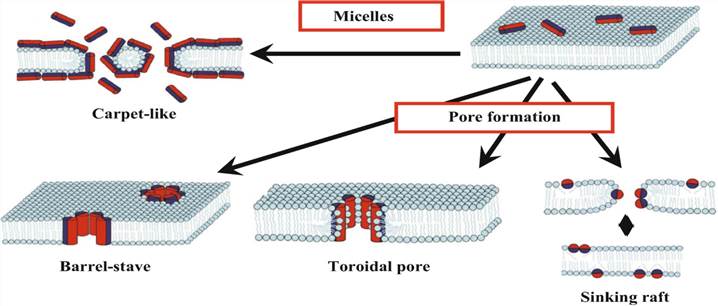 Figure 2. The main mechanism of bactericidal activity of cationic antimicrobial peptides. (Fillion et al., 2018)
Figure 2. The main mechanism of bactericidal activity of cationic antimicrobial peptides. (Fillion et al., 2018)
- Interaction analysis of antimicrobial peptides and model lipid membranes
Analysis of interaction between AMBP and intracellular target.
High-resolution 1H, 15N HSQC spectroscopy of micellar binding peptide to analyze the mechanism of membrane disturbance.
Analysis of non-layered phases (such as isotropic, cubic, and hexagonal phases).
Analysis of the influence of antimicrobial peptides on the orientation order and conformation of lipid polar head groups.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
References
- Vogel, H. J, et al. Characterization of antimicrobial and host-defense peptides by NMR spectroscopy. Modern Magnetic Resonance. 2018, 2055-2079.
- Fillion, M, et al. Solid-state NMR studies of the interactions and structure of antimicrobial peptides in model membranes. Modern Magnetic Resonance. 2018, 617-634.
