The structural biology field continues to evolve as scientists work to understand increasingly complex biological molecules. From membrane proteins to intricate molecular assemblies, these research challenges shape how we develop new medicines and comprehend disease pathways. Modern structural analysis methods, especially cryo-electron microscopy (cryo-EM) and X-ray crystallography, have transformed drug discovery. These techniques reveal atomic-detail snapshots of proteins, allowing researchers to design drugs with precision, verify therapeutic targets, and refine promising compounds. By combining insights from both methods, scientists can accelerate the development of targeted treatments while gaining deeper insights into the fundamental workings of biomacromolecules.
Overview of Cryo-EM and X-Ray Crystallography
What Is X-Ray Crystallography
Since its development, X-ray crystallography has stood at the forefront of molecular visualization, offering scientists a window into the atomic world of proteins and other biological molecules. When X-rays strike a crystallized sample, they scatter off the ordered molecular arrays in distinctive patterns. Scientists then decode these patterns to build detailed atomic maps, much like reconstructing a 3D puzzle from its shadows.
The power of this technique lies in its remarkable precision - routinely achieving resolutions finer than 2 Å, about a fifth of the width of a carbon atom. This extraordinary detail reveals the intimate architecture of molecules: how atoms connect to form chemical bonds, how protein chains fold into functional shapes, and exactly where drug molecules dock with their targets. Such atomic-level understanding has been instrumental in countless scientific breakthroughs, from designing better medicines to understanding the molecular basis of life itself.
What Is Cryo-EM
Scientists have made remarkable strides in visualizing life's molecular machinery through cryo-EM. This technique captures molecular snapshots by flash-freezing biological samples in extremely thin ice layers, preserving their natural shape and structure. Using powerful electron microscopes, researchers take thousands of pictures of these frozen molecules from different angles. Advanced computer programs then stitch these 2D snapshots together, much like assembling a 3D jigsaw puzzle, to reveal the molecule's complete structure.
What makes cryo-EM particularly valuable is its ability to study challenging biological structures in their natural state. Unlike older methods that required extensive sample preparation or crystallization, cryo-EM works with molecules in their near-native environment. This gentle approach allows scientists to observe biological molecules as they exist in nature, providing unprecedented insights into how they function within living systems.
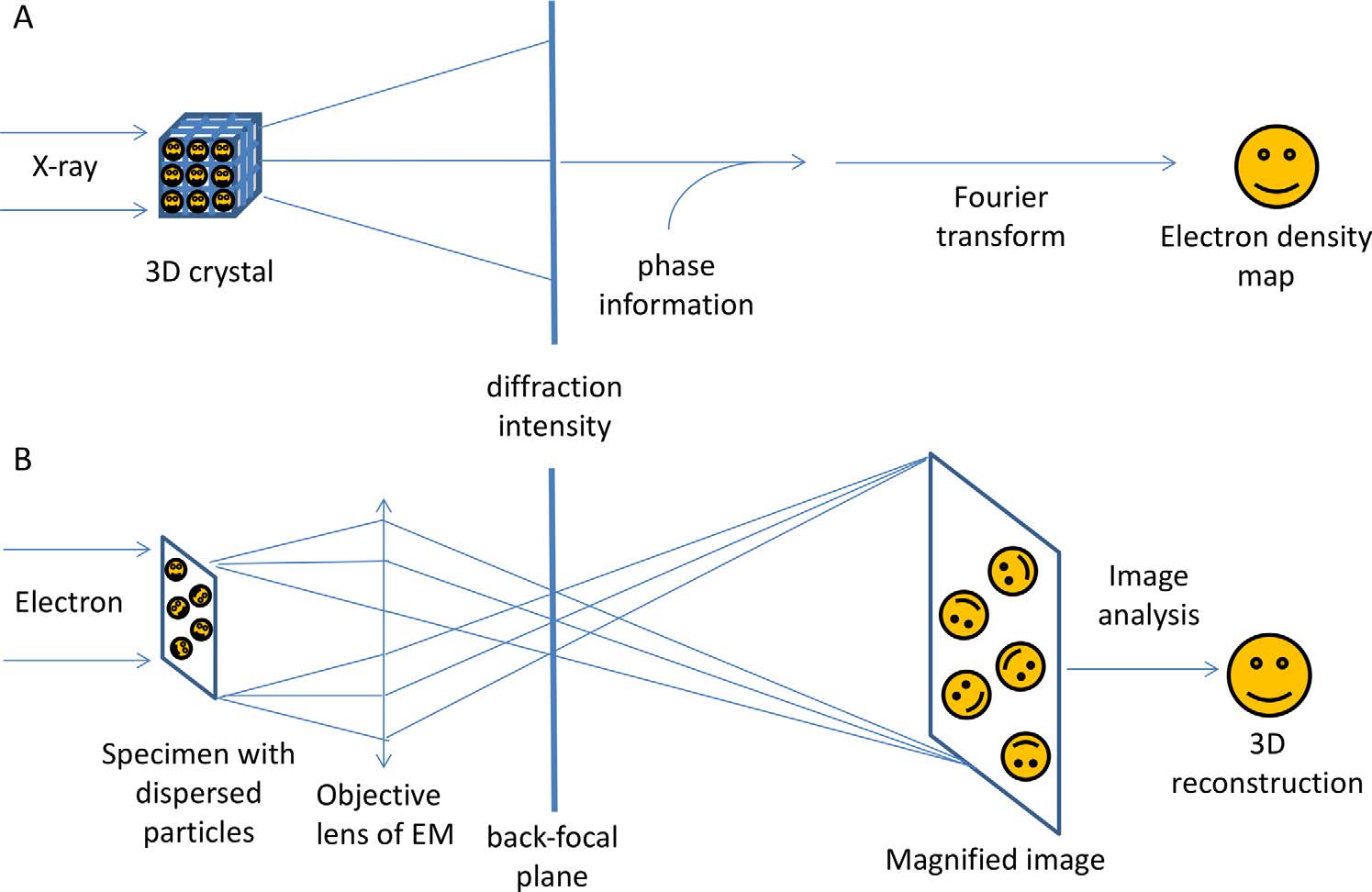 Figure 1. A comparison of the technical principles of X-ray crystallography and single-particle cryo-EM. X-ray crystallography uses diffraction patterns from molecules in a 3D lattice, facing a phase problem due to the lack of X-ray lenses. Cryo-EM directly images molecules using electrons, reconstructing density maps from 2D projections and providing diffraction patterns via the back-focal plane of the objective lens. (Wang H W, et al., 2017)
Figure 1. A comparison of the technical principles of X-ray crystallography and single-particle cryo-EM. X-ray crystallography uses diffraction patterns from molecules in a 3D lattice, facing a phase problem due to the lack of X-ray lenses. Cryo-EM directly images molecules using electrons, reconstructing density maps from 2D projections and providing diffraction patterns via the back-focal plane of the objective lens. (Wang H W, et al., 2017)
Key Advantages of Cryo-EM and X-ray Crystallography
Cryo-EM and X-ray crystallography represent complementary approaches to structural determination, each with distinct advantages:
Cryo-EM excels in:
- Analyze large molecular complexes
- Study proteins in their native states
- Capture multiple conformational states
- Require minimal sample amounts
- Examine challenging targets like membrane proteins
X-ray Crystallography offers:
- Atomic resolution structures
- High precision for small molecules
- Well-established data processing pipelines
- Robust validation methods
Choosing Between Cryo-EM and X-Ray Crystallography
Sample-Based Method Selection Criteria
Understanding your sample's intrinsic properties is crucial for selecting the most appropriate structural analysis method, as each technique has distinct advantages based on molecular characteristics and sample availability.
| Property | Cryo-EM | X-ray Crystallography |
| Molecular Size | Optimal >100 kDa | Optimal <100 kDa |
| Structural Stability | Flexible/Dynamic acceptable | Requires rigid structure |
| Sample Amount | 0.1-0.2 mg | >2 mg typically |
| Sample Purity | Moderate heterogeneity acceptable | High homogeneity required |
| Protein Type | Ideal for membrane proteins & complexes | Best for soluble proteins |
Project-Specific Requirements Analysis
Project success depends heavily on aligning method selection with specific research goals, timeline constraints, and available resources, while considering the desired resolution and data quality requirements.
| Factor | Cryo-EM | X-ray Crystallography |
| Resolution Range | Typically 2.5-4.0Å | Up to 1.0Å possible |
| Timeline | Weeks typically | Weeks to months |
| Data Collection | Hours to days | Minutes to hours |
| Cost Considerations | High microscope costs | Synchrotron access needed |
| Initial Results | Faster screening possible | Crystal optimization required |
Technical and Operational Considerations
The technical infrastructure, available expertise, and operational capabilities of your research environment play vital roles in determining the feasibility and potential success of each method.
| Aspect | Cryo-EM | X-ray Crystallography |
| Sample Preparation | Vitrification optimization | Crystal growth & optimization |
| Equipment Access | High-end microscope needed | Synchrotron access required |
| Data Processing | Intensive computing needed | Established pipelines |
| Expertise Required | EM & image processing | Crystallography & diffraction |
| Analysis Capability | Multiple conformations | Atomic precision |
Technical Deep Dive: Cryo EM vs X-ray Crystallography
Resolution Achievements and Limitations
- Breaking Resolution Barriers: While X-ray crystallography routinely achieves atomic resolution below 1Å, cryo-EM has revolutionized structural biology by reaching near-atomic resolutions (2-3Å) for challenging macromolecular complexes.
- Resolution Determining Factors: Each method's resolution depends on distinct factors - crystal order and diffraction quality for X-ray crystallography, versus particle alignment accuracy and detector sensitivity for cryo-EM.
| Resolution Aspect | Cryo-EM | X-ray Crystallography |
| Maximum Resolution | 2-3Å | Sub-1Å possible |
| Typical Resolution | 3-4Å | 1.5-2.5Å |
| Resolution Factors | - Sample quality - Ice thickness - Microscope stability |
- Crystal quality - Diffraction power - Data completeness |
| Resolution Limitations | Beam-induced damage | Crystal packing constraints |
Sample Preparation Pipeline
- Initial Processing Requirements: X-ray crystallography demands highly pure, homogeneous samples capable of crystal formation, while cryo-EM tolerates some heterogeneity but requires precise vitrification.
- Critical Quality Control: Success in both methods hinges on sample quality optimization - crystal perfection for X-ray studies and ice thickness control for cryo-EM analysis.
| Stage | Cryo-EM Sample Preparation | X-ray Crystallography |
| Initial Processing | Minimal purification | Extensive purification |
| Critical Steps | - Grid optimization - Vitrification conditions - Ice thickness control |
- Crystal screening - Optimization - Cryoprotection |
| Time Requirements | Days to weeks | Weeks to months |
| Key Challenges | - Ice quality - Sample concentration |
- Crystal quality - Crystal size |
Data Collection Strategies
- Acquisition Approaches: Cryo-EM involves collecting thousands of particle images under low-dose conditions, while X-ray crystallography requires high-quality diffraction patterns from well-ordered crystals.
- Equipment Specifications: Both methods rely on sophisticated instrumentation - high-end electron microscopes for cryo-EM and synchrotron radiation sources for advanced X-ray studies.
Data Processing Workflows
- Computational Requirements: Cryo-EM demands extensive computational resources for image processing and 3D reconstruction, while X-ray crystallography follows well-established diffraction data processing pipelines.
- Software Ecosystems: Both fields have developed robust software solutions, with cryo-EM experiencing rapid advances in automated processing and X-ray crystallography maintaining refined analysis tools.
| Aspect | Cryo-EM | X-ray Crystallography |
| Collection Time | Hours to days per dataset | Minutes to hours per dataset |
| Data Volume | Terabytes | Gigabytes |
| Processing Steps | - Motion correction - CTF estimation - Particle picking - 3D reconstruction |
- Data integration - Phasing - Model building - Refinement |
| Computing Needs | High-performance computing required | Standard workstation sufficient |
Timeline Considerations
- Project Duration: While initial screening may be faster with cryo-EM, both methods require significant time investment for optimization and high-resolution structure determination.
- Resource Allocation: Success in either method depends on balancing available expertise, equipment access, and computational resources throughout the project lifecycle.
Optimizing Method Selection for Specific Applications
Membrane Protein Structural Analysis
- Native Environment Preservation: Cryo-EM enables the study of membrane proteins in their near-native lipid environments, while X-ray crystallography provides atomic details of stable constructs through carefully optimized crystallization conditions.
- Complex Formation Understanding: The ability to visualize membrane protein complexes in different functional states provides crucial insights into their biological mechanisms and regulatory processes.
| Cryo-EM Advantages | X-ray Crystallography Considerations |
|
|
Large Protein Complex Studies
- Size-Dependent Capabilities: Cryo-EM particularly excels in analyzing large protein complexes without size limitations, offering insights into assembly mechanisms and subunit interactions that might be disrupted in crystallization.
- Resolution-Structure Relationship: While both methods can achieve high resolution, the choice between them often depends on the complex's stability, size, and conformational heterogeneity.
| Cryo-EM Superiority | X-ray Crystallography Applications |
|
|
Dynamic Structure Visualization
- Conformational Flexibility Analysis: Cryo-EM's ability to capture multiple conformational states in a single dataset provides unique insights into protein dynamics, complementing X-ray crystallography's atomic-resolution snapshots of stable states.
- Functional State Determination: Understanding protein dynamics through both methods offers comprehensive views of molecular mechanisms, from broad conformational changes to precise atomic movements.
| Cryo-EM Capabilities | X-ray Crystallography Contributions |
|
|
Select Service
Drug Discovery Applications
- Target-Specific Approach: Each method offers unique advantages in drug development - cryo-EM excels in visualizing large drug targets and conformational changes, while X-ray crystallography provides precise atomic details of drug-binding pockets.
- Structure-Based Drug Design: The complementary use of both techniques enhances drug discovery by providing comprehensive structural information across different scales and states of drug-target interactions.
- Screening and Optimization: While X-ray crystallography remains crucial for high-throughput ligand screening, cryo-EM is increasingly valuable for understanding drug effects on challenging targets like membrane proteins and large complexes.
| Cryo-EM Impact | X-ray Crystallography Strengths |
|
|
Each method offers unique advantages for specific research questions, with cryo-EM excelling in analyzing challenging targets and dynamic systems, while X-ray crystallography provides atomic precision for stable structures and small molecules. Successful structural biology programs often leverage both techniques complementarily to maximize research outcomes.
Both cryo-EM and X-ray crystallography offer unique advantages in structural biology research, and the choice between them depends on your specific research needs. At Creative Biostructure, whether you're studying membrane proteins, large complexes, or conducting drug discovery research, our experienced team can help you select and implement the most appropriate method for your project. Our state-of-the-art facilities and expertise in both techniques ensure high-quality structural analysis results. Contact us to discuss your research needs and discover how we can support your project success.
References
- Wang H W, Wang J W. How cryo‐electron microscopy and X‐ray crystallography complement each other. Protein Science. 2017, 26(1): 32-39.
- Callaway E. Revolutionary cryo-EM is taking over structural biology. Nature. 2020, 578(7794): 201-202.

-1.jpg)