What is Cryo-EM
Cryogenic Electron Microscopy (Cryo-EM) is an advanced biological imaging technology that can resolve the 3D formation of biological macromolecules by preserving specimens under cryogenic conditions and imaging them using an electron microscope. Its working principle is to quickly freeze the sample at an extremely low temperature to preserve its intrinsic state, and then examine and evaluate it using a Transmission Electron Microscope (TEM) or a Scanning Electron Microscope (SEM). This technology is particularly suitable for studying delicate biological samples that are sensitive to electron beams, such as viruses, protein complexes, and organelles.
Brief Overview of Cryo-EM's Importance
Cryo-EM is a powerful tool with diverse applications across multiple fields:
- Structural Biology: A core technique for resolving detailed 3D architectures of biomolecules, including membrane proteins, viruses, enzymes, and multiprotein complexes, providing crucial insights into biological processes.
- Drug Discovery: Facilitates structure-based drug design (SBDD) by revealing precise target protein structures, accelerating new drug development.
- Virus Research: Enables detailed structural analysis of pathogens like dengue and Zika viruses, aiding in vaccine development and understanding infection mechanisms.
- Material Science: Used to study radiation-sensitive materials at the atomic level, such as lithium-ion battery components, advancing material innovation.
Cryo-EM continues to revolutionize scientific research, drug development, and material science, with its impact expected to grow as technology advances.
Basic Principles of Cryo-EM
Key Components of Cryo-EM
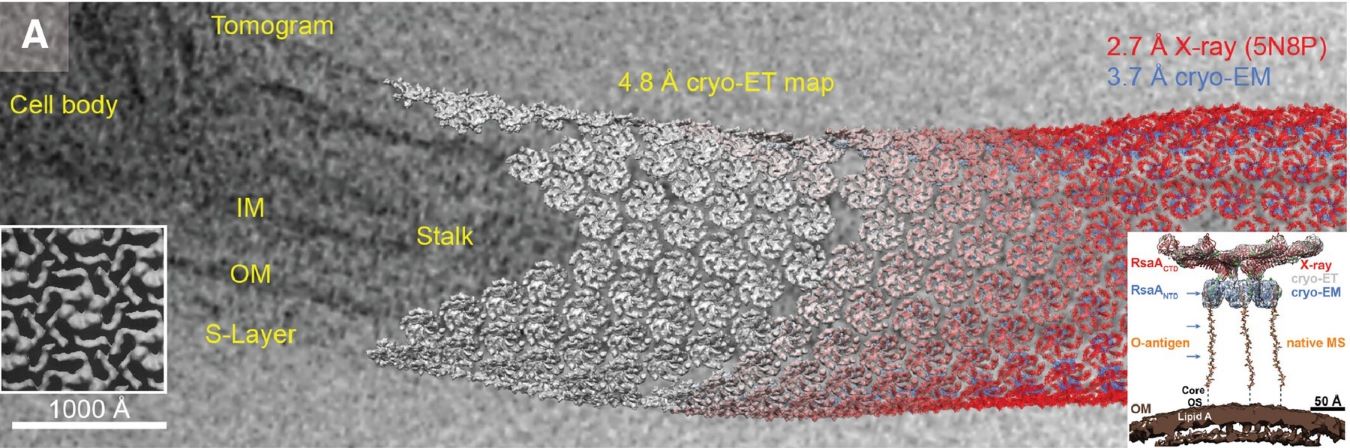
The core components of a cryo-EM setup include:
- Electron Source: High-energy electron beams are generated, often using a field emission gun (FEG) to achieve high-resolution imaging.
- Cryo-Holder: The sample is placed on a grid and frozen in vitreous ice. The cryo-holder maintains the sample at extremely low temperatures (~ -180°C) throughout the imaging process.
- Electron Optics: Electrons pass through the sample, and electromagnetic lenses focus and magnify the image.
- Direct Electron Detectors: These high-speed, highly sensitive detectors capture scattered electrons, enabling precise data acquisition.
The combination of these components allows for high-resolution imaging of biological samples without the need for crystallization.
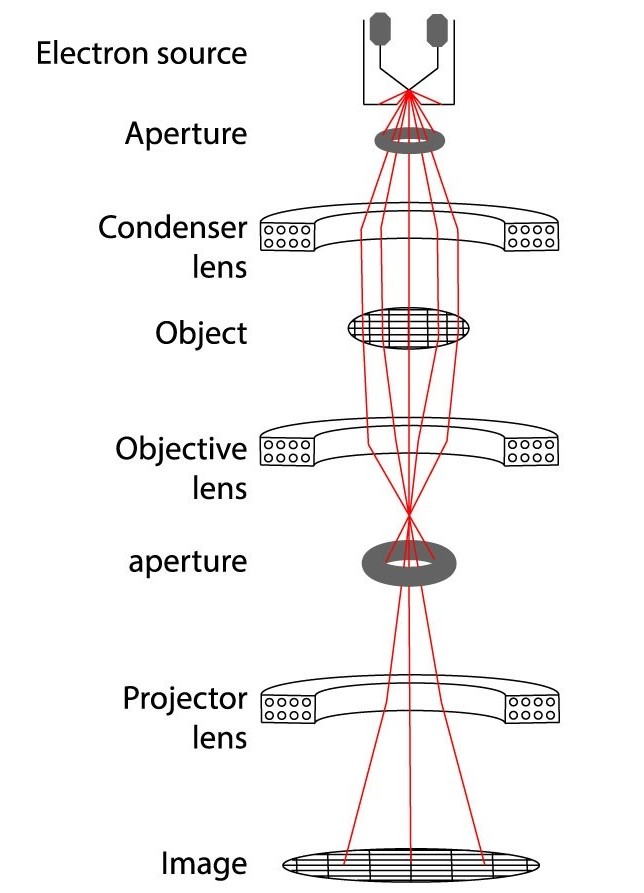 Figure 1. Schematic representation of an electron microscope. (Raimondi, et al., 2022)
Figure 1. Schematic representation of an electron microscope. (Raimondi, et al., 2022)
How Does Cryo-EM Work
1. Sample preparation
The core of Cryo-EM is to quickly freeze the sample to maintain its structure close to the physiological state. The sample is usually suspended in liquid ethane and quickly immersed in liquid nitrogen for vitrification to form an ice glass state, thereby avoiding the formation of ice crystals and mechanical damage to the sample.
2. Imaging process
Under low temperature conditions, the sample is placed in the sample chamber of the TEM. When the electron beam passes through the sample, it scatters with the atoms in the sample to form a scattering pattern. These scattering patterns are captured by the electron detector and converted into digital images.
3. Data processing and reconstruction
By collecting a large number of two-dimensional images at different angles, these images are reconstructed using computational algorithms to finally generate a three-dimensional model. This process relies on complex mathematical modeling and computing resources.
 Figure 2. Workflow of single-particle cryo-EM: sample vitrification, TEM imaging, motion correction, particle selection, 2D averaging, and 3D reconstruction. (Chung and Kim, 2017)
Figure 2. Workflow of single-particle cryo-EM: sample vitrification, TEM imaging, motion correction, particle selection, 2D averaging, and 3D reconstruction. (Chung and Kim, 2017)
Key Differences from Conventional Transmission Electron Microscopy
In contrast to conventional TEM, where samples are exposed to room-temperature conditions, cryo-EM preserves biological structures by rapidly freezing them, typically in liquid ethane. This prevents water within the sample from crystallizing, keeping the sample hydrated and in a more intrinsic state.
| Aspect | Cryo-EM | TEM |
| Sample Preparation | Utilizes rapid freezing techniques to preserve samples in a near-native state, avoiding common TEM procedures such as fixation, staining, or dehydration. This minimizes artifacts and structural alterations. | Requires fixation, staining, or dehydration to enhance electron density, which may alter the sample's native structure. |
| Resolution & Scope | Achieves atomic-level resolution (approximately 3 Å) due to technological advancements, enabling the detailed structural analysis of complex biomolecules, such as protein complexes and membrane proteins. | Limited by the optical diffraction limit (approximately 0.2 nm), making it challenging to resolve high-resolution structures of large molecular complexes. |
| Sample Types & Range | Suitable for a wide range of biological samples, including viruses, proteins, and cellular organelles. No strict size limitations, capable of resolving molecules ranging from tens of Daltons to over 500 kDa. | Limited applicability for small and large molecule. |
| Imaging Environment | Imaging is conducted at cryogenic temperatures (-150°C to -196°C) to reduce thermal motion and preserve the dynamic properties of the sample. | Typically performed at room temperature or slightly higher, which may lead to thermal motion and structural changes in the sample. |
| Data Collection & Analysis | Employs single-particle analysis (SPA) or subtomogram averaging to reconstruct 3D models from numerous 2D images. This approach allows for the analysis of heterogeneous samples and dynamic processes. | Relies on direct observation of 2D images for structural analysis, lacking support for dynamic processes and heterogeneous samples. |
Cryo-EM Techniques
With the advancement of technology, Cryo-EM has developed multiple branch methods, which are mainly used in the fields of biological macromolecules, cell structure and drug development. Its core branches include the following three categories:
Single Particle Analysis (SPA)
Principle: Collect a large number of randomly oriented single particles (such as protein molecules) in two-dimensional projection images and use algorithms to reconstruct them in three dimensions.
Features: Suitable for uniform and stable biological macromolecules (such as viruses, ribosomes, etc.), currently can achieve atomic resolution (<3 Å).
Application: Widely used to analyze the structure of protein complexes, virus capsids, etc., and provide molecular details for drug design.
Cryo-Electron Tomography (Cryo-ET)
Principle: Obtain two-dimensional images at different angles by tilting the sample and reconstructing the three-dimensional structure, which is particularly suitable for studying the in-situ protein dynamics in cells.
Features: Combining the high resolution of single particle analysis with the spatial information of the cell environment, it fills the gap between optical microscopy and atomic resolution technology.
Application: Reveal the working mechanism of complex structures such as nuclear pore complexes and membrane-bound ribosomes.
Microcrystal Electron Diffraction (MicroED)
Principle: Deducing atomic structure based on electron diffraction data of tiny crystals (<200 nm).
Features: Suitable for samples that are difficult to form large crystals (such as small molecule drugs, membrane proteins), and can extract high-resolution information from heterogeneous mixtures.
Application: Accelerate the development of new drugs and biomaterials.
Other Related Technologies and Extensions
Electron Crystallography: used for two-dimensional crystal structure analysis (such as rhodopsin) in the early days, and now partially replaced by MicroED.
Technical synergy: Cryo-EM is often combined with immunolabeling and three-dimensional reconstruction algorithms (such as non-uniform optimization algorithms) to improve the accuracy of complex sample analysis.
Select Service
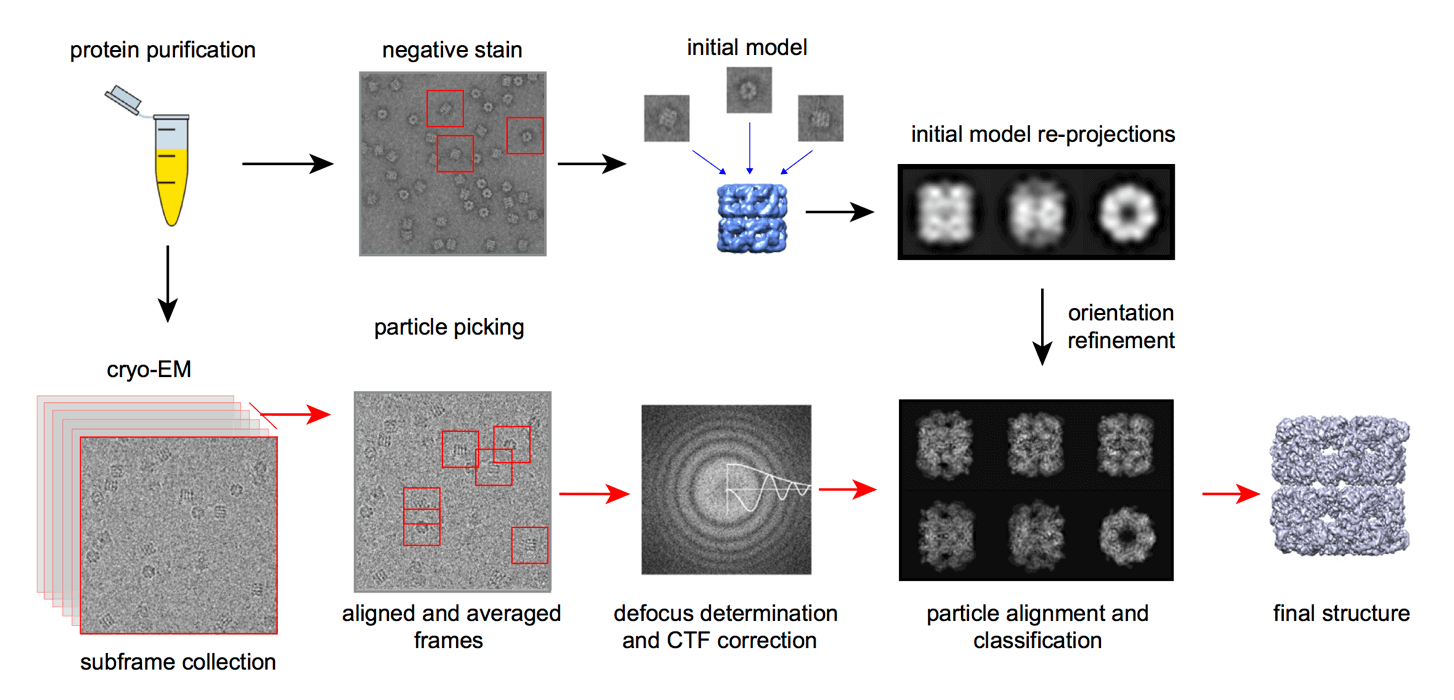
The Cryo-EM Workflow
A. Sample Preparation
- Sample solution
The sample needs to be highly purified (such as recombinant expression or endogenous extraction) to ensure the structural homogeneity and integrity of the biomacromolecule or complex. Low-purity samples may lead to nonspecific particles or background interference. The sample needs to be dispersed in a suitable buffer to avoid protein denaturation or aggregation.
- Vitrification
The sample solution is converted into a glassy ice layer by rapid freezing to avoid crystallization and damage to the structure. This process requires strict control of the temperature gradient and freezing rate. Gas-liquid interface problems may lead to protein aggregation or orientation preference, which needs to be alleviated by improved grid design (such as carbon film modification) or high-pressure freezing technology. Diagnostic Cryo-EM evaluation should be performed immediately after sample preparation to screen particle distribution, purity and low-resolution three-dimensional reconstruction potential.
B. Microscopy Process
- Sample processing
The sample needs to be kept at low temperature (liquid nitrogen temperature) throughout the process to avoid thawing or ice crystal formation. Use a high-precision cryotransfer system (such as cryo-FIB or HPF) to process large-volume samples. Samples need to be pre-processed before data acquisition, including light microscopy screening, area marking (such as grid square positioning) and dose optimization.
- Microscope calibration
Includes precise adjustment of the accelerator, objective lens, and projection system to ensure electron beam parallelism and image resolution. CTF (contrast transfer function) parameters need to be calibrated in real time to compensate for optical distortion during imaging.
C. Image Processing and 3D Reconstruction
- Image correction and quality improvement
Motion correction: correct the drift of particles under the action of electron beam and restore high-resolution information.
Dose weighting: give priority to the use of high-quality data of early low-dose frames to reduce the impact of electron beam damage.
CTF estimation and correction: compensate for objective lens chromatic aberration and spherical aberration to optimize image signal-to-noise ratio.
Computational denoising: remove background noise through algorithms to improve particle recognition accuracy.
- Particle identification and 3D reconstruction
Particle selection: Manual or semi-automatic algorithms (such as cryoSPARC, RELION) extract individual particles from micrographs.
2D classification: Classify particles by 2D similarity, screen high-quality particles and remove abnormal states.
3D reconstruction: Iteratively optimize particle orientation through projection matching algorithm and construct preliminary 3D model.
Model optimization: Combine atomic models (such as X-ray diffraction data) for structural refinement, and finally generate high-resolution 3D density map.
Types of Cryo-EM Microscopy
Cryo-Transmission Electron Microscopy (Cryo-TEM)
1. Imaging Technology
Basic principle: Based on the electron beam penetrating the sample to form a transmission image, it needs to be operated at a low temperature (close to the liquid nitrogen temperature zone) to maintain the original state of the sample and avoid structural damage caused by ice crystal formation.
Key advantages: Allows the sample to be observed in a fully hydrated state without chemical fixation or staining, reducing artificial artifacts. Applicable to in situ imaging of biological macromolecules (such as protein complexes, viruses), nanomaterials (such as MOFs, HOFs) and liquid samples.
2. 3D Reconstruction Technology
Cryo-TEM achieves three-dimensional structure analysis through a variety of methods:
Single particle analysis (SPA): Collect two-dimensional projection images of a large number of purified macromolecular particles, and generate high-resolution three-dimensional models after calculation and averaging (resolution can reach the atomic level).
Cryo-electron tomography (cryo-ET): Continuously image samples at tilted angles and reconstruct three-dimensional structures through back-projection algorithms. It is suitable for complex biological samples (such as organelles, viruses).
MicroED: Reconstruct the structure using small crystal diffraction images and optimize the resolution in combination with tilt angles.
Other techniques: such as electron differential absorption contrast (EPA) and nanoscale CT scanning, further enhance imaging details.
Cryo-Scanning Electron Microscopy (Cryo-SEM)
1. Surface Imaging and Analysis
Cryo-SEM generates images by scanning an electron beam to excite secondary electrons (SE) or backscattered electrons (BSE):
Surface Detail Observation: Suitable for high-resolution surface morphology analysis of hydrated samples (e.g., cells, biological tissues), avoiding dehydration and structural collapse caused by the vacuum environment in conventional SEM.
Elemental Analysis: Combined with energy-dispersive X-ray spectroscopy (EDS), it can detect elemental distribution in samples. However, careful selection of coating materials (e.g., carbon, aluminum) is required to avoid interference.
2. Sample Preparation and Operation
Rapid Freezing and Transfer: Samples are immobilized using liquid nitrogen or high-pressure freezing methods and maintained in a low-temperature vacuum environment using anti-contamination devices (e.g., cryo-transfer shuttles).
Surface Treatment Techniques:
Freeze Fracturing and Planing: Exposes internal structures, combined with sublimation to remove frost contamination and preserve the original morphology.
Coating: Enhances conductivity and improves imaging quality, typically using low atomic number materials (e.g., platinum).
3. Technical Features
Advantages: Enables observation of liquid samples and termination of dynamic processes (e.g., gel solidification). Capable of imaging electron beam-sensitive samples (e.g., certain polymers).
Limitations: Conventional Cryo-SEM is a two-dimensional technique; limited three-dimensional information (thickness ≤ 200–300 nm) can only be obtained when combined with electron tomography.
Comparison Summary of Cryo-TEM and Cryo-SEM
| Characteristic | Cryo-TEM | Cryo-SEM |
| Core Function | 3D structure reconstruction (SPA, cryo-ET) | Surface morphology and elemental analysis |
| Typical Applications | Biomacromolecules (proteins, viruses), materials science | Biological tissues, liquid samples |
| Technical Challenges | High-resolution reconstruction algorithms | Sample freezing and contamination prevention |
| Resolution | Atomic-level (single-particle analysis) | Nanoscale surface details |
Advantages and Disadvantages of Cryo-EM
Advantages of Cryo-EM
1. High Resolution with Minimal Sample Distortion
- Cryo-EM utilizes rapid vitrification, freezing samples at liquid nitrogen temperatures to preserve their near-physiological state in solution, avoiding structural distortions caused by traditional chemical fixation or dehydration.
- Direct electron detectors and advanced image processing algorithms enable atomic-resolution imaging (e.g., 2.3 Å), facilitating the structural analysis of complex biomolecules such as membrane proteins and ion channels.
- Unlike X-ray crystallography, Cryo-EM does not require sample crystallization, overcoming challenges associated with difficult-to-grow or stringent crystallization conditions.
2. Broad Sample Compatibility
- Applicable to a wide range of samples, from single proteins (e.g., 64 kDa catalase) to whole cells and tissues.
- Particularly suitable for studying membrane proteins, dynamic conformational heterogeneity, and structural changes (e.g., drug-binding states).
- Innovations such as hydrophobin film (HFBI-film) and graphene oxide grids enhance imaging stability for small-molecule samples.
Limitations of Cryo-EM
1. Technical Complexity and High Cost
- Instrumentation Dependency: Requires specialized equipment such as cryo-holders, direct electron detectors, and low-dose imaging systems, making acquisition and upgrades highly expensive.
- Operational Challenges: Sample preparation demands precise control over ice thickness (typically 50–100 nm), and low-dose electron imaging results in a low signal-to-noise ratio, necessitating advanced image processing expertise.
- Radiation Sensitivity: High-resolution imaging requires strict electron dose limitations, increasing the demand for stable low-temperature conditions and sample integrity.
2. Low Throughput and Sample Constraints
- High sample consumption (~0.1 mg per experiment), with limited reusability, making large-scale screening or serendipitous discoveries challenging.
- Resolution constraints for small molecules (<100 kDa): While some small protein structures (e.g., 43 kDa kinase subunit) have been resolved, throughput remains lower compared to larger macromolecular complexes.
- Thick samples prepared via high-pressure freezing require additional sectioning or focused ion beam thinning, introducing potential artifacts.
Cryo-EM Applications
1. Drug Development and Targeted Therapy
Structure-Based Drug Design (SBDD)
Cryo-EM enables atomic-resolution visualization of protein-drug complexes, providing critical structural insights for designing small-molecule inhibitors, antibodies, and molecular glues. For example, analyzing the binding mode of γ-secretase with its inhibitors facilitates the optimization of drug specificity. Additionally, integrating Cryo-EM data with artificial intelligence algorithms (e.g., deep learning) accelerates lead compound screening and optimization.
Disease Mechanism Studies
In oncology, Cryo-EM has elucidated the three-dimensional structures and dynamic conformational changes of key oncogenic proteins, such as mitochondrial DNA transcription complexes, offering a structural basis for inhibitor development. Furthermore, Cryo-EM enables the visualization of organelle structural changes (e.g., Golgi apparatus, mitochondria), aiding the development of targeted drug delivery systems.
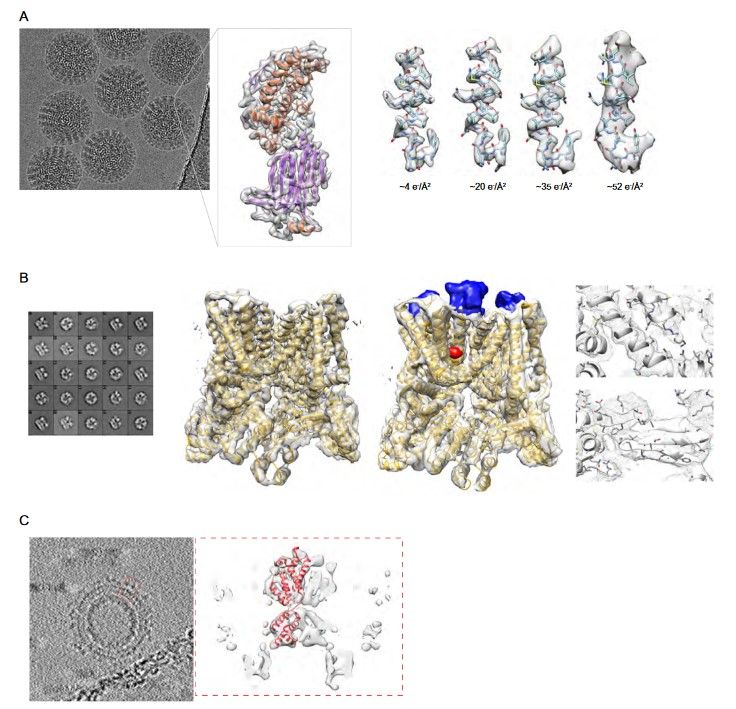 Figure 3. Examples of cryo-EM macromolecular reconstructions (A) An average of aligned frames of the rotavirus double-layered particle and 3D reconstruction of an extracted subunit. On the right are shown the effects of electron damage on a segment of the polypeptide chain. (B) Structure of the TrpV ion channel with class averages on the left, structure without (EMD-5778) and with ligands (blue and red; EMD-5777) in the middle, details of fitted α-helices and β-strands on the right. (C) Subtomogram averaging of Gag protein from RSV retrovirus. On the left, averaged slices through the tomogram of immature viral particles (EMD-3102). In the right dashed box, subtomogram average of a Gag subunit with the fitted secondary structure (EMD-3101). (Carroni and Saibil, 2016)
Figure 3. Examples of cryo-EM macromolecular reconstructions (A) An average of aligned frames of the rotavirus double-layered particle and 3D reconstruction of an extracted subunit. On the right are shown the effects of electron damage on a segment of the polypeptide chain. (B) Structure of the TrpV ion channel with class averages on the left, structure without (EMD-5778) and with ligands (blue and red; EMD-5777) in the middle, details of fitted α-helices and β-strands on the right. (C) Subtomogram averaging of Gag protein from RSV retrovirus. On the left, averaged slices through the tomogram of immature viral particles (EMD-3102). In the right dashed box, subtomogram average of a Gag subunit with the fitted secondary structure (EMD-3101). (Carroni and Saibil, 2016)
2. Virology and Infectious Disease Control
Viral Structure Analysis
Cryo-EM single-particle analysis has revealed high-resolution viral capsid structures, such as the prefusion conformation of the SARS-CoV-2 spike protein (3.5 Å resolution), directly informing the design of neutralizing antibodies and vaccines. Similarly, structural studies of influenza and Ebola viruses heavily rely on Cryo-EM.
Disinfectant and Vaccine Development
Structural information on viral surface proteins aids in the optimization of antiviral drugs (e.g., interferon analogs) and provides a basis for antigen epitope selection in vaccine design.
3. Cell Biology and Organelle Research
Dynamic Observation of Organelles
Cryo-EM overcomes structural distortions caused by dehydration and chemical fixation in conventional electron microscopy, allowing for the clear visualization of native membrane structures such as mitochondrial outer membrane pores (MOMP) and Golgi stacks. For instance, by visualizing the formation process of outer membrane vesicles (OMVs), Cryo-EM facilitates research on mitochondrial permeability regulation mechanisms.
Functional Analysis of Protein Complexes
Cryo-EM plays a crucial role in elucidating the structures of large macromolecular machines, such as ribosomes and nuclear pore complexes. For example, it has revealed discrete RNA folding intermediates, advancing our understanding of RNA processing and ribonucleoprotein assembly.
Select Service
Cryo-EM continues to revolutionize our understanding of life at the molecular level. From unraveling complex protein structures to visualizing viral particles, from identifying drug targets to examining cellular organelles in unprecedented detail, Cryo-EM is opening new frontiers in biological research.
At Creative Biostructure, we combine cutting-edge cryo-EM technology with expert knowledge to support your research goals. Our experienced team and state-of-the-art facilities are ready to assist with your structural biology projects, whether in drug development, viral research, or fundamental scientific discovery. Ready to advance your research with cryo-EM? Contact us to discuss how our comprehensive cryo-EM services can accelerate your project's success.
References
- Chung, J.-H., & Kim, H. M. (2017). The nobel prize in chemistry 2017: High-resolution cryo-electron microscopy. Applied Microscopy, 47(4), 218–222.
- Carroni, M., & Saibil, H. R. (2016). Cryo electron microscopy to determine the structure of macromolecular complexes. Methods, 95, 78–85.
- Raimondi, V., Grinzato, A., Raimondi, V., & Grinzato, A. (2022). A basic introduction to single particles cryo-electron microscopy. AIMS Biophysics, 9(1), 5–20.
- Wang, Z., Zhang, Q., & Mim, C. (2021). Coming of age: Cryo-electron tomography as a versatile tool to generate high-resolution structures at cellular/biological interfaces. International Journal of Molecular Sciences, 22(12), 6177.

-1.jpg)