A Brief Introduction to Transmission Electron Microscopy (TEM)
The Transmission Electron Microscope (TEM) is a powerful imaging technique that uses electrons to visualize the internal structure of thinly sliced samples. Unlike Scanning Electron Microscope (SEM), which provide surface images, TEMs transmit electrons through a sample, allowing internal structures to be examined at the atomic or molecular level. TEMs are widely used in biological, chemical, and materials science research because of their exceptional resolution, allowing detailed imaging of cells, organelles, viruses, and molecular complexes.
History of TEM
The development of the TEM began in the early 20th century. In 1931, German physicist Ernst Ruska and electrical engineer Max Knoll built the first prototype of an electron microscope, for which Ruska received the Nobel Prize in Physics in 1986. Their work was based on the realization that electron wavelengths were much shorter than those of visible light, allowing for far greater resolution than light microscopes. By 1933, Ruska's team had developed a more advanced version capable of imaging objects at a much higher resolution than any light microscope of the time.
Following World War II, TEM technology was further refined and commercialized. Companies such as Siemens and Hitachi began producing TEMs for scientific use. Over time, advances in electron optics, vacuum technology, and digital imaging greatly improved the performance of the TEM, making it a critical tool for structural biology and materials science.
 Figure 1. The duplicate of an early TEM on display at the Deutsches Museum in Munich, Germany.
Figure 1. The duplicate of an early TEM on display at the Deutsches Museum in Munich, Germany.
Basic Principles of TEM
The principle of TEM is based on the interaction between electrons and matter. An electron beam is accelerated by a high voltage (typically 80–300 kV) and focused onto a thin specimen. As the electrons pass through the sample, they interact with its atoms, either scattering or transmitting. Electrons transmitted or scattered at different angles are collected and magnified by electromagnetic lenses to form an image on a fluorescent screen or digital camera.
The contrast in TEM images arises from differences in electron density within the sample, where denser regions scatter more electrons, resulting in darker areas on the image. The resolution of TEM can reach sub-nanometer levels, making it a powerful tool for visualizing the internal structures of cells, proteins, and nanomaterials.
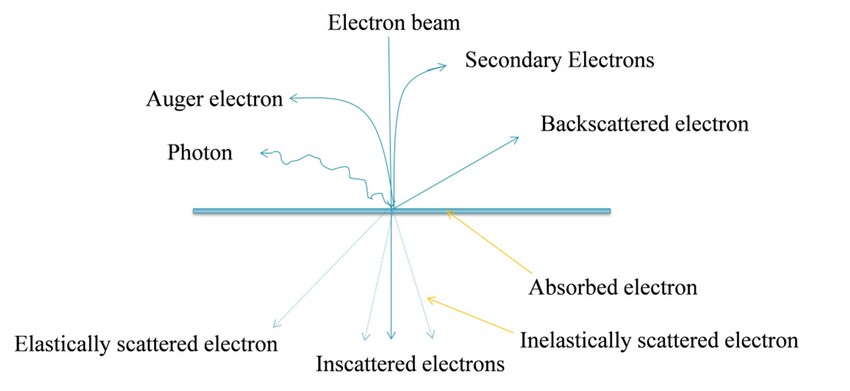 Figure 2. Basic principle of Transmission Electron Microscopy (TEM) (Ahmed et al., 2015).
Figure 2. Basic principle of Transmission Electron Microscopy (TEM) (Ahmed et al., 2015).
Instrumentation of TEM
A TEM consists of several key components that enable the imaging process:
Electron Source: The electron gun generates a beam of electrons by heating a filament (usually made of tungsten) or using field emission. The electrons are accelerated toward the specimen using high voltages, often between 100 kV and 300 kV.
Condenser Lenses: These electromagnetic lenses focus the electron beam onto the specimen. By controlling the beam's intensity and focus, the condenser lenses allow the electron beam to be finely tuned for optimal imaging.
Objective Lens: After passing through the specimen, the electrons are further focused by the objective lens, forming the primary magnified image.
Projection Lens: The final magnification of the image is achieved through projection lenses, which enlarge the image for display on a fluorescent screen or digital detector.
Vacuum System: A high vacuum is required in the TEM chamber to prevent electrons from scattering by air molecules. This ensures that the electron beam interacts only with the specimen.
Detectors: TEMs often include detectors, such as a fluorescent screen or a charge-coupled device (CCD) camera, to capture and display the image.
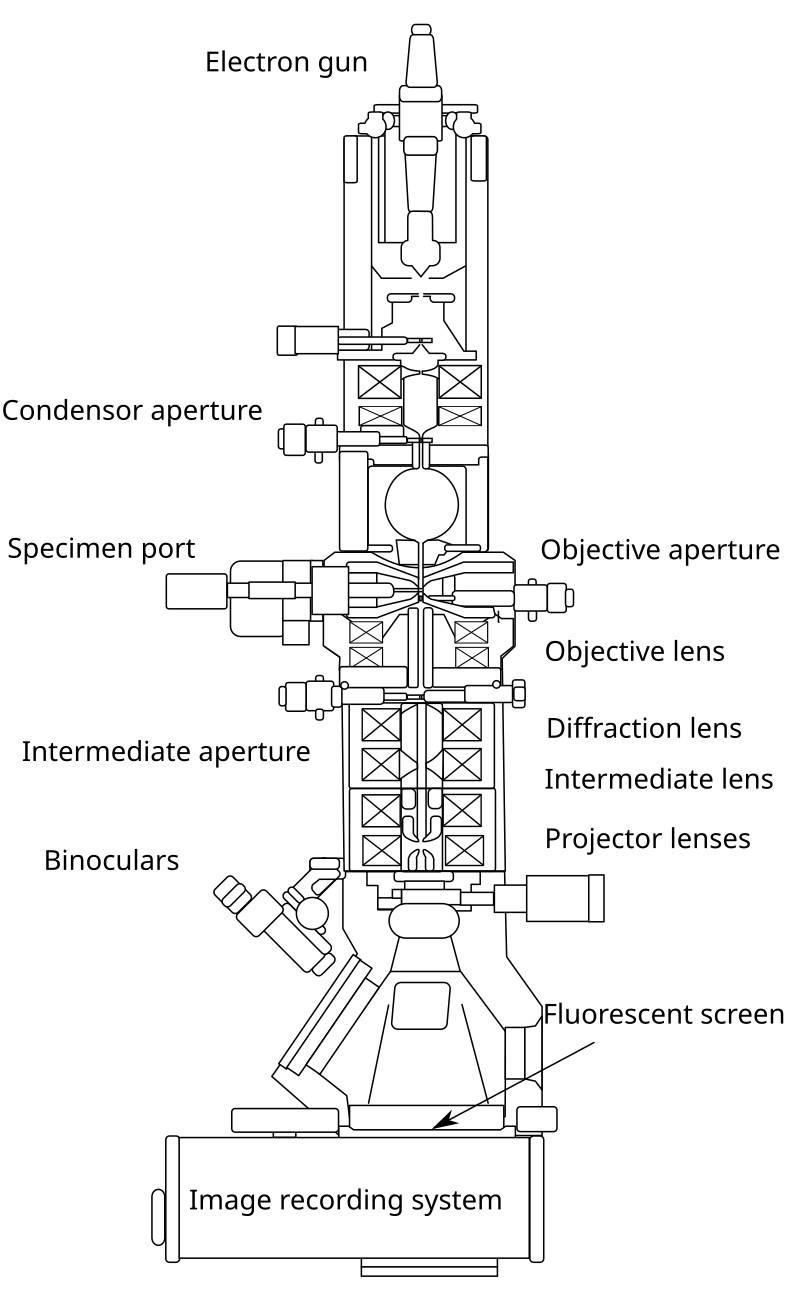 Figure 3. Layout of optical components in a basic TEM.
Figure 3. Layout of optical components in a basic TEM.
Preparation of Specimen for Visualization by TEM
Sample preparation for TEM is critical because the specimen must be extremely thin (typically less than 100 nm thick) to allow electrons to pass through it. The following are the typical steps involved:
Fixation: Biological samples are often fixed using chemical agents like glutaraldehyde or formaldehyde to preserve their structure.
Dehydration: Samples are dehydrated using a series of ethanol or acetone washes to remove water, which could disrupt the vacuum environment.
Embedding: The sample is embedded in a resin, such as epoxy or acrylic, to provide mechanical stability during sectioning.
Sectioning: Ultra-thin sections of the sample are cut using an ultramicrotome. These sections are typically about 50–100 nm thick.
Staining: Since biological specimens are composed mainly of low atomic number elements (carbon, hydrogen, oxygen), they do not scatter electrons well. Heavy metals like lead, osmium, or uranium are used to stain the specimen, increasing electron contrast.
Mounting: The stained sections are mounted onto a copper grid for insertion into the TEM chamber.
Bright-field vs. Dark-field Mode of TEM
Transmission Electron Microscopy (TEM) operates in two basic imaging modes: bright-field and dark-field, which are based on how electron interactions with the sample are visualized. Both modes provide complementary views, with bright-field offering broad contrast and dark-field highlighting specific structural features.
Bright-field Mode
In bright-field TEM, the sample is illuminated with a parallel beam of electrons, and the image is formed by transmitting electrons that pass through the sample without significant scattering. Areas where electrons pass through easily appear bright, while regions with thicker or denser structures scatter electrons more, appearing darker. This mode is typically used for observing general structural features like tissue, cellular components, or crystallographic patterns in thin specimens.
Dark-field Mode
In dark-field TEM, the scattered electrons are used to form the image, while the unscattered electrons are blocked. This creates an image where the scattered areas appear bright against a dark background. Dark-field mode enhances contrast for features that weakly scatter electrons, making it useful for highlighting specific structures, defects, or crystallographic details that may be difficult to detect in bright-field mode.
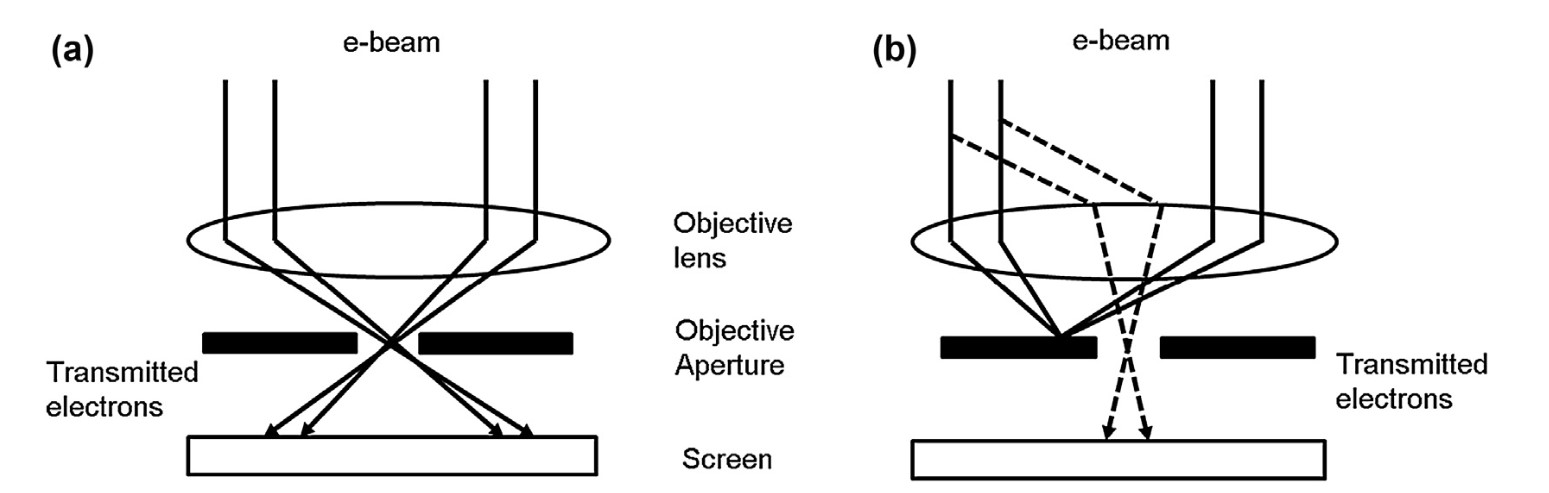 Figure 4. Transmitted and diffracted electrons for (a) bright-field and (b) dark-field images in transmission electron microscope (Tang and Yang, 2017).
Figure 4. Transmitted and diffracted electrons for (a) bright-field and (b) dark-field images in transmission electron microscope (Tang and Yang, 2017).
Cryo-EM and Cryo-ET
Cryo-Electron Microscopy (Cryo-EM) is a variation of TEM in which samples are frozen at cryogenic temperatures (around -180°C) to preserve their natural structure without the need for staining or fixation. Cryo-EM provides near-atomic resolution, making it particularly useful for studying large biomolecular complexes in their native, hydrated states. Samples are vitrified (rapidly frozen) in a thin layer of ice, minimizing damage and preserving the native state. Cryo-EM is widely used in structural biology to determine the 3D structures of proteins, viruses, and other macromolecular complexes.
Cryo-Electron Tomography (Cryo-ET) is a specialized form of cryo-EM that captures multiple 2D images of a sample from different angles and reconstructs them into a 3D model. This technique provides high-resolution 3D imaging, although generally at a lower resolution than single-particle cryo-EM. Similar to cryo-EM, sample preparation for cryo-ET involves vitrification of the sample but is specifically designed for thicker samples such as cells. Cryo-ET is used to study the 3D architecture of cells, organelles, and complex molecular structures in situ, providing a more comprehensive view than cryo-EM alone.
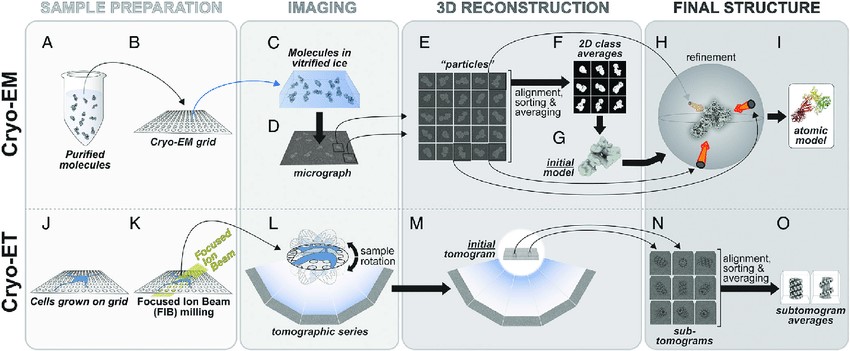 Figure 5. The cryo-EM and cryo-ET pipelines. (A-I) In cryo-EM, Purified molecules (A) are placed on EM grids (B), frozen (C) and imaged using transmission EM (D). Individual particles (E) are used to generate 2D class averages (F), which are then used to build an initial 3D model (G) that is then further refined (H). Depending on the resolution of the final cryo-EM map, an atomic model can be built into it (I). (J-O) In cryo-ET, cells are grown on EM grids (J) and, when targeting thicker areas, FIB milling is used to generate thin slices of cells (K). The milled cells are then imaged using transmission EM and a tomographic series is obtained (L, M). Sections of a tomogram containing the same structure are segmented to generate sub-tomograms (N) and these are averaged to generate sub-tomogram averages (O). See text for a more detailed explanation of the pipelines (Leschziner and Reck‐Peterson, 2021).
Figure 5. The cryo-EM and cryo-ET pipelines. (A-I) In cryo-EM, Purified molecules (A) are placed on EM grids (B), frozen (C) and imaged using transmission EM (D). Individual particles (E) are used to generate 2D class averages (F), which are then used to build an initial 3D model (G) that is then further refined (H). Depending on the resolution of the final cryo-EM map, an atomic model can be built into it (I). (J-O) In cryo-ET, cells are grown on EM grids (J) and, when targeting thicker areas, FIB milling is used to generate thin slices of cells (K). The milled cells are then imaged using transmission EM and a tomographic series is obtained (L, M). Sections of a tomogram containing the same structure are segmented to generate sub-tomograms (N) and these are averaged to generate sub-tomogram averages (O). See text for a more detailed explanation of the pipelines (Leschziner and Reck‐Peterson, 2021).
Advantages of TEM
TEM offers several key advantages:
Table 1. Advantages of TEM technique.
| High Resolution | TEM offers resolutions down to the sub-nanometer level, making it one of the most powerful techniques for studying small structures. |
| Detailed Internal Imaging | Unlike SEM, which provides surface images, TEM allows for the visualization of internal structures. |
| Versatility | TEM can be applied to a wide range of specimens, from biological tissues to inorganic materials. |
Disadvantages of TEM
At the same time, there are several disadvantages of TEM:
Table 2. Disadvantages of TEM technique.
| Complex Sample Preparation | Preparing samples for TEM is labor-intensive and requires expertise, particularly for biological specimens. |
| Sample Damage | The high-energy electron beam used in TEM can cause damage to sensitive specimens, especially biological samples. |
| Vacuum Requirement | The need for a high vacuum limits the types of samples that can be analyzed, as hydrated or living samples must undergo extensive preparation. |
Applications of TEM
In structural biology, TEM is indispensable for visualizing the internal architecture of cells, viruses, and macromolecules. Some key applications include:
Cellular Ultrastructure: TEM is used to study the fine structure of cells, including organelles such as mitochondria, the endoplasmic reticulum, and nuclei. TEM can reveal the arrangement of cellular components at the molecular level, aiding in understanding cell function and pathology.
Virus Structure: TEM has been instrumental in elucidating the morphology of viruses. The high resolution of TEM allows researchers to study viral capsid structures, viral entry mechanisms, and interactions with host cells.
Protein Complexes: Cryo-electron microscopy (Cryo-EM), a specialized form of TEM, has revolutionized the study of protein structures. Cryo-EM enables the imaging of proteins in their native, hydrated state, allowing for the determination of near-atomic resolution structures of protein complexes and molecular assemblies.
Nanotechnology: TEM is widely used in nanotechnology to study the morphology, crystallography, and composition of nanoparticles, nanowires, and other nanomaterials.
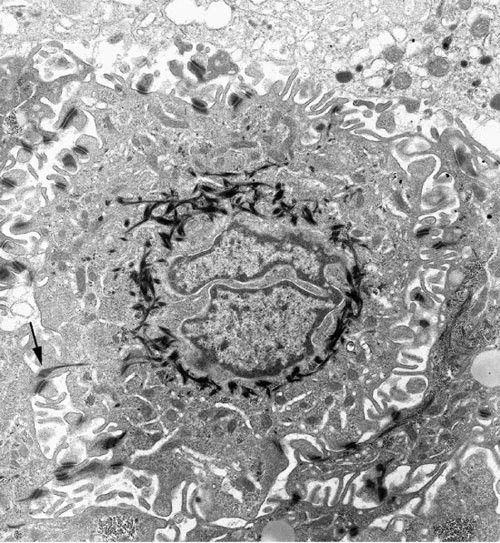 Figure 6. A squamous cell in a squamous cell carcinoma imaged by TEM. The deeply indented nucleus is surrounded by electron-dense bundles of cytokeratin. The irregular cell surface has many desmosomes with cytokeratin tails (arrow). × 9,000 (Graham and Orenstein, 2007).
Figure 6. A squamous cell in a squamous cell carcinoma imaged by TEM. The deeply indented nucleus is surrounded by electron-dense bundles of cytokeratin. The irregular cell surface has many desmosomes with cytokeratin tails (arrow). × 9,000 (Graham and Orenstein, 2007).
In summary, Transmission Electron Microscopy (TEM) is a powerful technique that has transformed our understanding of the microscopic world. With its ability to visualize internal structures at high resolutions, TEM is an essential tool in structural biology, materials science, and nanotechnology. Despite its advantages, TEM requires careful sample preparation and operates under stringent conditions such as high vacuum and thin samples. The difference between TEM and SEM lies in their applications - while SEM focuses on surface imaging, TEM excels in providing detailed internal images, contributing to significant advances in various scientific fields. For more information on SEM vs. TEM, please visit the page: Transmission Electron Microscopy vs. Scanning Electron Microscopy.
Creative Biostructure offers Transmission Electron Microscope (TEM) analysis services designed to provide the precise, high-resolution insights essential to advancing your structural biology research. With state-of-the-art technology and a team of experienced professionals, we provide the support you need to unlock molecular and cellular secrets. Partner with us to enhance your research and accelerate your discoveries. Contact us today to discuss your TEM analysis needs and learn how we can contribute to the success of your projects.
References
- Membrane Characterization. Chapter Transmission electron microscopy (TEM), pp. 145–159, Elsevier, 2017.Tang, C. Y., & Yang, Z.
- Ahmed, S., Messali, Z., Ouahabi, A., Trepout, S., Messaoudi, C., & Marco, S. (2015). Nonparametric denoising methods based on contourlet transform with sharp frequency localization: Application to low exposure time electron microscopy images. Entropy, 17(5), 3461–3478.
- Burghardt, R. C., & Droleskey, R. (2006). Transmission electron microscopy. Current Protocols in Microbiology, 3(1).
- Engel, A. (2022). Biological applications of the scanning transmission electron microscope. Journal of Structural Biology, 214(2), 107843.
- Graham, L., & Orenstein, J. M. (2007). Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nature Protocols, 2(10), 2439–2450.
- Leschziner, A. E., & Reck‐Peterson, S. L. (2021). Structural Biology of LRRK2 and its Interaction with Microtubules. Movement Disorders, 36(11), 2494–2504.