Custom MemPro™ BCL2/adenovirus E1B 19 kD-Interacting Proteins (BNIPs)
Creative Biostructure has developed custom MemPro™ gene-to-structure services for BNIPs. BNIPs are a class of BH3-only Bcl-2 proteins belonging to the BCL2 family and contain a single BCL2 homology3 (BH3) domain each. They interact with adenovirus E1B 19kD proteins and play critical roles in two major degradation processes: apoptosis and autophage. There are 3 classes of BNIPs: BNIP1, BNIP2 and BNIP3. They were first identified by a yeast two-hybrid screening in 1994. And there are also BCL2/adenovirus E1B 19 kD-Interacting Proteins like BNIPLs.
- BNIP1, a 228-amino acid protein, mainly located in endoplasmic reticulum (ER) and nuclear membrane and interacting with RNF185 and Bcl-2.
- BNIP2, a 315-amino acid protein, mainly located in endoplasmic reticulum (ER) and nuclear membrane and interacting with ARHGAP1, Bcl-2 and CDC42.
- BNIP3, a 194-amino acid protein, mainly located in mitochondrial outer membrane and interacting with Bcl-2 and STEAP3.
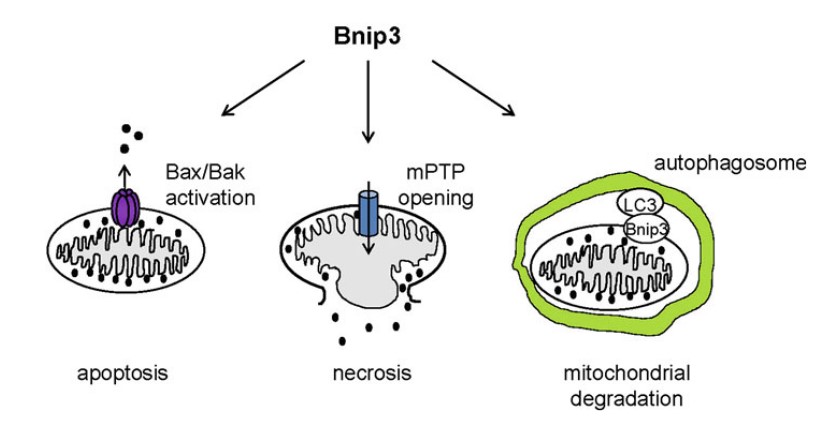
Figure 1. Bnip3 regulates cell death of cardiac myocytes (Pediatr Cardiol. 2011)
BNIP1 interacting with adenovirus E1B 19-kDa protein is actually a component of the syntaxin 18 complex. BNIP1 has low but important sequence similarity with yeast Sec20p. BNIP1 interacts with α-SNAP via BH3 domain. Functionally, BNIP1 may be a key factor in ER membrane fusion. Researches show that over-expression of BNIP1 results in ER aggregation and loss of BNIP1 induces the disruption of the network. BNIP1 is a pro-apoptotic protein. Its promoter has a functional hypoxia response element which can be activated by E2F and HIF-1α. BNIP2 has been implicated in the suppression of cell death. It can interact with Cdc42 to regulate cell elongation and membrane protrusions. BNIP3 now is the most widely studied member of BNIP family. Structurally, it contains four major domains: the PEST domain, proline (P), glutamic acid (E), serine (S), threonine (T) and aspartic acid (D), towards the N-terminus targeting BNIP3 for degradation; the BH3 domain adjacent to the PEST domain; a CD domain and a carboxy-terminal transmembrane (TM) domain targeting BNIPs to the mitochondria. BNIP3 has been found to be involved in various cellular functions involving many different cellular pathways. It can induce apoptosis and pro-death, on the other hand it also acts as a pro-survival protein. BNIP3 relates with mitochondrial dysfunction resulting in cardiac abnormalities, neurological and disorders and rheumatoid arthritis. BNIP3 has also been implicated in hypoxia-induced cell death and it is a potent inducer of autophage in various cells.
Creative Biostructure can provide custom MemPro™ gene-to-structure services for membrane proteins.
Nagarjuna Vasagiri and Vijay Kumar Kutala. Structure, function, and epigenetic regulation of BNIP3: a pathophysiological relevance.
A˚sa B. Gustafsson. Bnip3 as a Dual Regulator of Mitochondrial Turnover and Cell Death in the Myocardium. Pediatr Cardiol. 2011 Mar;32(3):267-74.