Structural Research of Gasdermin (GSDM) Family
Gasdermin (GSDM) proteins have emerged as key players in various inflammatory diseases and cancer. The study of their structure and function is crucial for understanding their role in cellular processes and for the development of potential therapeutic interventions. Among the GSDM family members, Gasdermin D (GSDMD) has gained significant attention due to its role in pyroptosis, a form of inflammatory cell death. The functional understanding of GSDMD has been greatly advanced by the elucidation of its structure and pore-forming mechanism.
Recent breakthroughs in cryo-electron microscopy (cryo-EM) have enabled researchers to visualize the structures of GSDMD pores and prepores at unprecedented resolutions. Notably, studies have reported the formation of 27-fold and 28-fold single-ring pores by the N-terminal fragment of GSDMA3 (GSDMA3-NT) at 3.8 Å and 4.2 Å resolutions, respectively. Additionally, a double-ring pore structure has been observed at 4.6 Å resolution. These structural insights have provided a basis for understanding the activities of mutant gasdermins, including those associated with cancer.
The cryo-EM structures of GSDMA3-NT pores revealed a 108-stranded anti-parallel β-barrel formed by two β-hairpins from each subunit, capped by a globular domain. Notably, a positively charged helix was identified to interact with the acidic lipid cardiolipin, suggesting a crucial role in membrane binding and pore formation. Membrane insertion of GSDMA3-NT induced radical conformational changes, resulting in the formation of long, membrane-spanning β-strands. Intriguingly, the double-ring pore structure exhibited an additional symmetric ring of GSDMA3-NT subunits that did not insert into the membrane, possibly representing a pre-pore state.
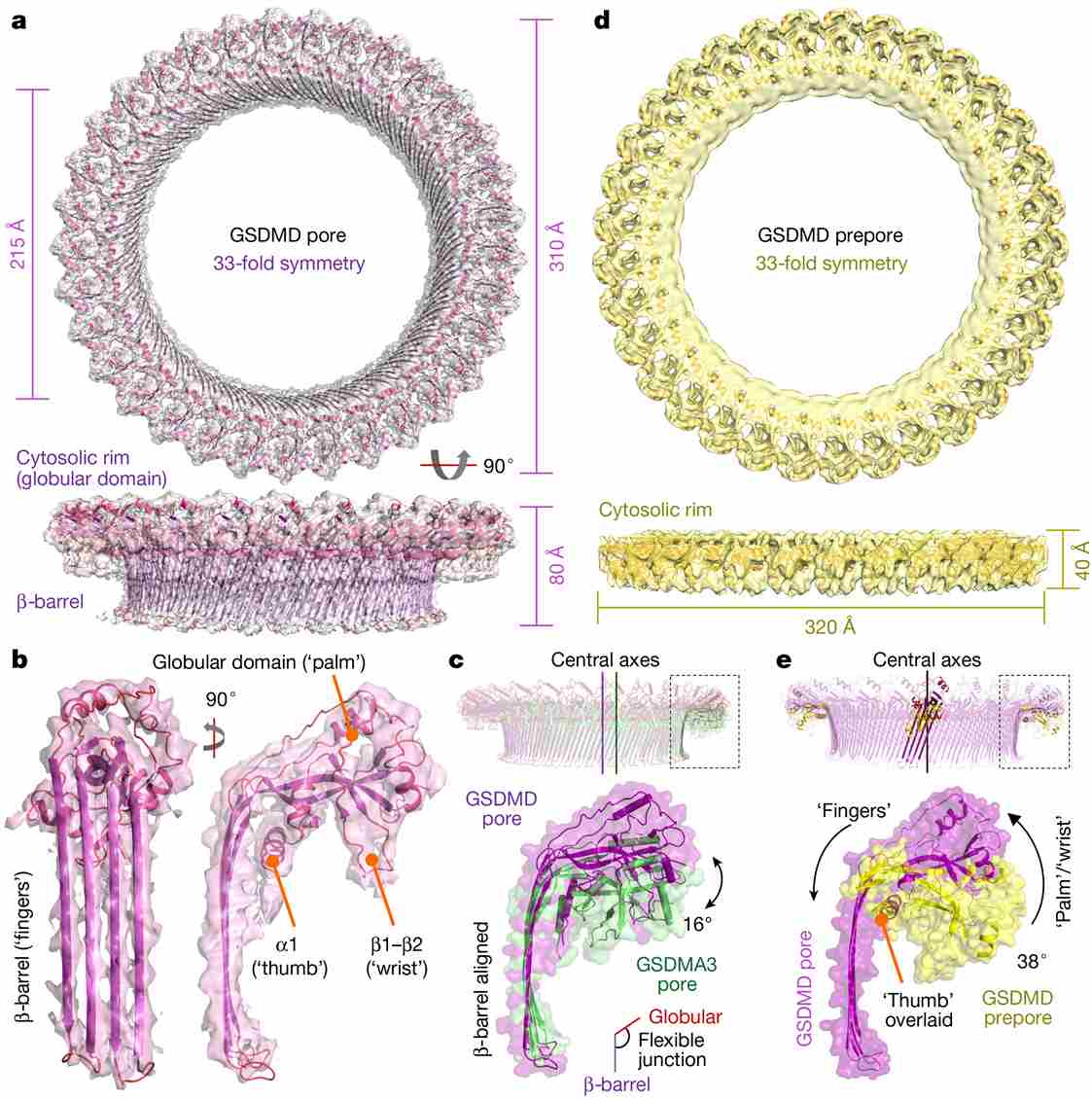 Figure 1. GSDMD architecture and conformational changes. (Xia S, et al., 2021)
Figure 1. GSDMD architecture and conformational changes. (Xia S, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| Gasdermin A3 membrane pore (expressed in Escherichia coli) | Mus musculus | Cryo-EM single particle analysis | 3.80 Å | 6CB8 |
| Gasdermin D pore (expressed in Escherichia coli) | Homo sapiens | Cryo-EM single particle analysis | 3.90 Å | 6VFE |
| GSDMB pore (expressed in Escherichia coli) | Homo sapiens | Cryo-EM single particle analysis | 4.96 Å | 8ET2 |
| Gasdermin B pore (expressed in Escherichia coli) | Homo sapiens | Cryo-EM single particle analysis | 3.17 Å | 8GTN |
Table 1. Structural Research of Gasdermin (GSDM) Family.
At Creative Biostructure, our commitment to excellence drives us to stay at the forefront of scientific advancements. Our state-of-the-art cryo-EM facility enables high-resolution imaging and structural determination of GSDM proteins. Our team of experienced scientists and technicians employ sophisticated data processing and analysis algorithms to elucidate the complex architectures of GSDM pores and prepores. We offer tailored experimental design and execution, ensuring accurate and reliable results.
In addition to cryo-EM, we offer a wide range of structural analysis techniques, including X-ray crystallography, NMR spectroscopy, and molecular modeling. These methods complement each other, enabling comprehensive structural characterization of GSDM proteins and their functional domains. Our integrated approach allows for a multidimensional understanding of the structure-function relationships underlying GSDM-mediated inflammatory processes. Contact us to embark on a journey of groundbreaking discoveries in structural biology.
References
- Ruan J, et al. Cryo-EM structure of the gasdermin A3 membrane pore. Nature. 2018, 557(7703): 62-67.
- Xia S, et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021, 593(7860): 607-611.
- Wang C, et al. Structural basis for GSDMB pore formation and its targeting by IpaH7. 8. Nature. 2023, 616(7957): 590-597.
- Zhong X, et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature. 2023, 616(7957): 598-605.