Atomic Force Microscope (AFM) for Exosome Characterization
Exosomes are molecular complexes composed of lipid-membrane vesicles, membrane proteins, other molecular surface modifications, and various contents inherited from parental cells, including RNA, proteins, and DNA. The relative differences between fluid dynamics and vesicle size are significant for exosomes. Cryogenic transmission electron microscopy (cryo-TEM) imaging is a gold standard technique, but characterizing exosomes remains a challenge due to the cost of the equipment, the expertise required for sample preparation, imaging, and data analysis, and the small number of particles observed in the images. In recent years, atomic force microscopy (AFM) has emerged as a widely used alternative method to generate a wide range of data on exosomes' three-dimensional geometry, size, and other biophysical properties.
What is the AFM?
AFM is a new type of equipment invented after the scanning tunneling microscope (STM) with high resolution at the atomic level. Its display resolution is on the order of nanometers, more than 1000 times higher than the optical diffraction limit. It is possible to probe physical properties including morphology in the nano region of various materials and samples in atmospheric and liquid environments or to perform direct nanomanipulation. The information is collected by "feeling" or "touching" the surface with a mechanical probe. Piezoelectric elements are moved minutely in response to (electronic) commands, enabling precise scanning.
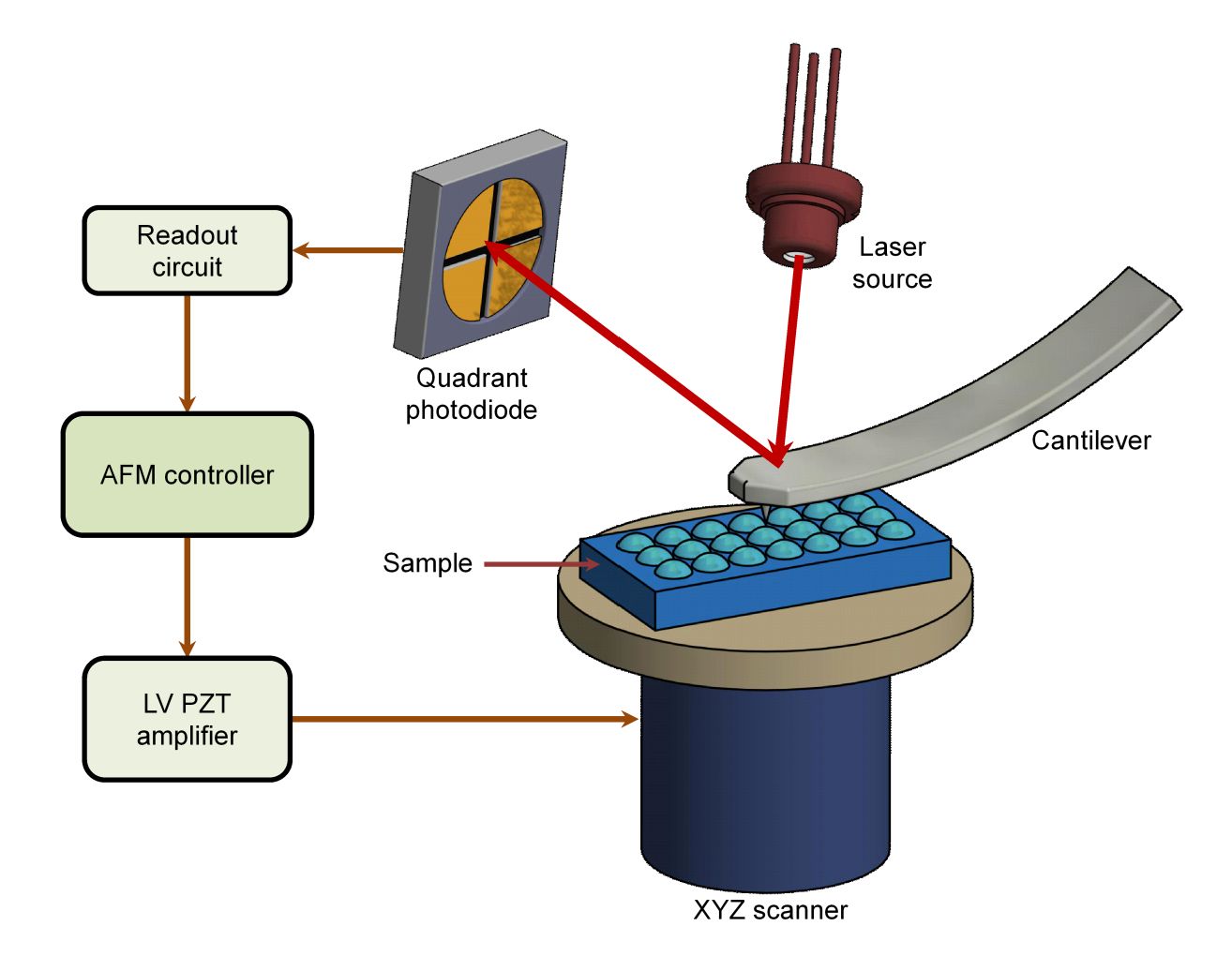 Figure 1. General schematic of the atomic force microscope (AFM). (Alunda BO, Lee YJ, 2020)
Figure 1. General schematic of the atomic force microscope (AFM). (Alunda BO, Lee YJ, 2020)
Mechanical characterization of exosomes is essential for elucidating their physiological and pathological roles. AFM has a wide range of applications in biology and is a commonly used method for the energetic characterization of submicron-sized exosomes. It provides high-resolution images of exosomes at the single-particle level under near-physiological conditions and allows for force spectroscopy. AFM imaging has been used to characterize the size and shape of individual natural exosomes and to reveal their mechanical properties.
How does AFM Work?
During AFM analysis, a microcantilever, which is extremely sensitive to weak forces, is fixed at one end, and the other end is a sharp-tipped probe, with the tip of the needle gently in contact with the sample surface. Due to the very weak repulsive force between atoms at the tip of the needle and atoms on the surface of the sample, the microcantilever with the tip of the needle will move perpendicularly to the surface direction of the sample, corresponding to the isotropy of the force between the tip of the needle and atoms on the surface of the sample, by controlling the constancy of such a force during the scanning time. The microcantilever with the tip will undulate in the direction perpendicular to the surface of the sample corresponding to the isotropic surface of the force between the tip and the sample surface. By using the optical detection method or tunneling current detection method, the change in the position of the microcantilever corresponding to each point of the scan can be measured, and information on the surface topography of the sample can be obtained.
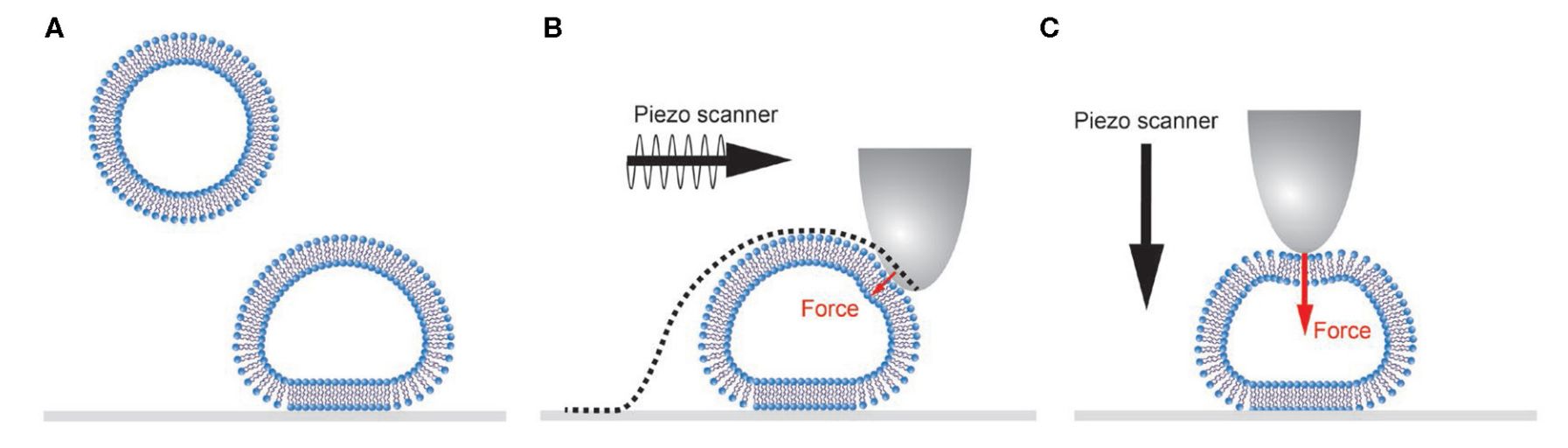 Figure 2. Schematic representation of single vesicle AFM experiments. (Vorselen D, et al., 2020)
Figure 2. Schematic representation of single vesicle AFM experiments. (Vorselen D, et al., 2020)
AFM Equipment Structure
- Force Detection Section
In an AFM system, a microcantilever is used to detect the amount of change in van der Waals forces between atoms. The cantilever is usually made of a silicon wafer or a silicon nitride wafer with a sharp tip that is used to detect the sample-tip interaction force.
- Position Detection Section
The cantilever oscillates when there is an interaction between the needle tip and the sample. The position of the reflected light when the laser is directed at the end of the microcantilever also changes as the cantilever oscillates. The offset is recorded using a laser spot position detector and converted into an electrical signal for signal processing by the SPM controller.
- Feedback System
After the signal is picked up by the detector, it is used as an internal adjustment signal in the feedback system, which drives the scanner to move appropriately so that the sample maintains a certain force on the tip of the needle. Finally, the surface characteristics of the sample are visualized.
What are the Operating Modes of AFM?
The operating modes of the AFM are categorized in terms of the form of force between the tip of the needle and the sample. There are 3 main modes of operation:
1) Contact Mode - Contact mode is the most direct imaging mode of AFM. AFM maintains close contact between the probe tip and the sample surface throughout the scanning and imaging process. The force exerted on the tip by the cantilever during scanning has the potential to damage the surface structure of the sample.
2) Non-Contact Mode - In non-contact mode, the cantilever oscillates at a distance of 5-10nm above the sample surface. The interaction between the sample and the tip of the needle is controlled by Van der Waals forces, the sample is not damaged and the tip of the needle is not contaminated, which makes it particularly suitable for researching the surface of delicate objects. The disadvantage of this mode of operation is that it is very difficult to realize it in a room-temperature atmosphere.
3) Tapping Mode - The percussion mode is intermediate between the contact and non-contact modes. The cantilever oscillates at its resonant frequency above the sample surface and the tip of the needle only periodically taps the surface briefly. The AFM's percussion mode is therefore one of the best choices when examining tender samples.
Application
With the development of science and technology, life sciences began to develop in a quantitative direction. Biological macromolecules have become the focus of research in most experiments, especially exosomes in cancer diagnosis and drug delivery. Because of the wide working range of AFM, biomedical samples can be directly imaged in the natural state with high resolution. Therefore, AFM has become one of the essential tools for researching biomedical samples and biomolecules. AFM applications mainly include:
- Surface morphology observation of biological cells.
- Observation and research of the structure and other properties of biomolecules.
- Observation of force spectrum curves between biomolecules.
We offer a range of exosomes isolated from cancer cell lines that can be used as positive controls for exosome isolation, characterization, and functional research.
| Cat No. | Product Name | Source |
| Exo-CH15 | HQExo™ Exosome-BLCL21 | Exosome derived from EBV transformed lymphoblastoid B cells (BLCL21 cell line) |
| Exo-CH08 | HQExo™ Exosome-BPH-1 | Exosome derived from human benign prostatic hyperplasia-1 (BPH-1 cell line) |
| Exo-CH22 | HQExo™ Exosome-HCT116 | Exosome derived from human colorectal carcinoma cell line initiated from an adult male (HCT116 cell line) |
| Exo-CH17 | HQExo™ Exosome-HT29 | Exosome derived from human adenocarcinoma (HT29 cell line) |
| Exo-CH18 | HQExo™ Exosome-LnCAP | Exosome derived from human prostate adenocarcinoma (LnCAP cell line) |
| Explore All Exosomes Isolated from Cancer Cell Lines | ||
What are the Highlights of AFM?
- The AFM provides a true 3D surface map.
- The AFM does not require any special treatment of the sample, such as copper or carbon plating, which can cause irreversible damage to the sample.
- The AFM works well at atmospheric pressure and even in liquid environments.
Creative Biostructure is a leading biological company specializing in providing high-quality exosome characterization services. We are constantly updating our techniques and methods based on the latest scientific findings to ensure the best service for our clients. Working with us, clients can be assured of precise, efficient, and superior exosome characterization results. If you are interested in our exosome products and exosome services, please feel free to contact us!
References
- Alunda BO, Lee YJ. Review: Cantilever-Based Sensors for High Speed Atomic Force Microscopy. Sensors. 2020. 20(17): 4784.
- Vorselen D, et al. Mechanical Characterization of Liposomes and Extracellular Vesicles, a Protocol. Front Mol Biosci. 2020. 7: 139.
- Skliar M, Chernyshev VS. Imaging of Extracellular Vesicles by Atomic Force Microscopy. J Vis Exp. 2019. (151): 10.3791/59254.
- Szatanek R, et al. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int J Mol Sci. 2017. 18(6): 1153.
