MemPro™ CAAX protease
Creative Biostructure provides custom MemPro™ gene-to-structure services for CAAX protease.
CAAX protease is an integral membrane protease located at the endoplasmic reticulum to catalyze proteins contain a CAAX motifs. CAAX proteins play essential roles in multiple signaling pathways which are identified by their characteristic post translational modification(s) on the C-terminal CAAX sequences: CAAX motifs (where C is cysteine, A is an aliphatic amino acid and X is any amino acid). These proteins include Ras superfamily of small GTPases, nuclear lamins, the γ-subunit of heterotrimeric GTPases, and several protein kinases and phosphatases. CAAX protease regulates the reactions of cysteine prenylation, -AAX tripeptide cleavage, and methylation of the carboxyl prenylated Cys residue. Its protease activity is mediated by the Ras and a-factor converting enzyme 1 (Rce1).
ZMPSTE24, also known as famesylated-protein converting enzyme 1 (FACE-1), is a CAAX protease that is essential for maturation of lamin A. ZMPSTE24 adopts a seven transmembrane a-helical barrel structure while on the nucleoplasmic side of the membrane, the chamber is sealed by a M48 zinc metalloprotease domain that similar to thermolysin. The seven transmembrane helices (TMHs) are packed in an antiparallel a-helical bundle, with the zinc metalloprotease fold inserted between TMH5 and TMH6.
Lamin A is one of three lamin proteins found in the lamina of differentiated cells. ZMPSTE24 or the Rce1 protease subsequently cleave the AAX residues from the C terminus of prelamin A (the CAAX cleavage reaction), followed by carboxymethylation of the farnesyl cysteine. ZMPSTE24 then cleaves an additional 15 residues from the C terminus of prelamin A. This removes the membrane-embedded farnesyl cysteine (fCys) and releases mature lamin A into the nucleoplasm. Mutation of ZMPSTE24 results in accumulation of farnesylated, membrane-associated prelamin A, which is called laminopathies.
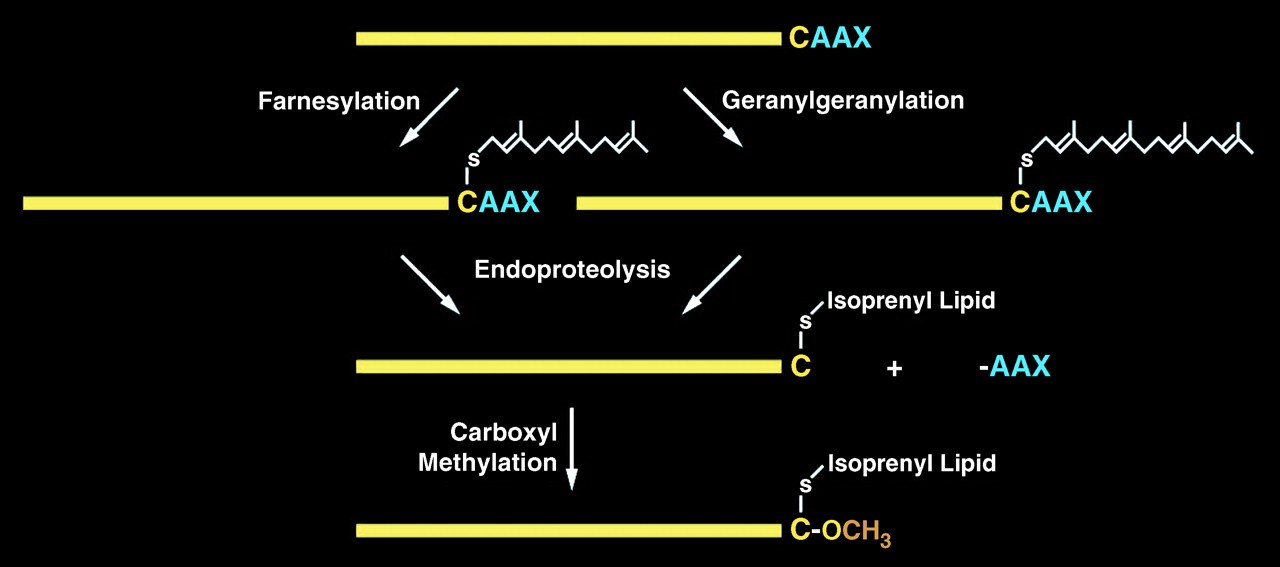
Figure 1. prelamin A endoprotelytic process
In humans, the severity of ZMPSTE24-dependent laminopathies correlates with the level of residual protease activity. The most severe ZMPSTE24-dependent laminopathies are neonatal lethal restrictive dermopathy (RD) and the premature aging (progeria) disease atypical Hutchinson-Gilford progeria syndrome (HGPS). Milder laminopathies—such as mandibuloacral dysplasia type B (MAD-B) (14) and, potentially, metabolic syndrome (MS)—have a range of symptoms including lipodystrophy (LD) and insulin resistance, bone and skin abnormalities, and cardiomyopathy. During normal aging, ZMPSTE24 expression decreases in vascular smooth muscle cells, which leads to accumulation of unprocessed prelamin A, disrupting mitosis and DNA damage repair. There is also evidence that ZMPSTE24 is inhibited by certain HIV protease inhibitors, and the side effects of taking these drugs resemble laminopathies.
For now, there is no cure of laminopathies and treatment is quite limited. Understanding of CAAX protease may help uncover the process of toxic progerin formation and lead to the development of targeted treatment.
Reference
Quigley A, Dong Y Y, Pike A C W, et al. The structural basis of ZMPSTE24-dependent laminopathies[J]. Science, 2013, 339(6127): 1604-1607.
Manolaridis I, Kulkarni K, Dodd R B, et al. Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1[J]. Nature, 2013, 504(7479): 301-305.