Labeling Exosomes by Lentivirus-Mediated CD63-GFP Expression
Exosomes are membrane-encapsulated extracellular vesicles carrying a variety of components (including nucleic acids, proteins, lipids metabolites, etc.) from the parent cell, with a diameter of 30-150nm and a lipid bilayer structure. Exosomes can be recognized and fused by cells, and are essential mediators of inter-cellular regulation, participating in the pathogenesis of cancer, neurodegenerative and inflammatory diseases, and affecting cellular functions in many ways. Labeling and tracking of exosomes can facilitate further research on their functions, including release, uptake, and biodistribution mechanisms, to explore the potential applications of exosomes as biomarkers and therapeutics.
Exosomes are naturally present in blood, urine, cerebrospinal fluid, and in the supernatant of cultured cells in vitro, and almost all types of cells can produce and release exosomes. Research has found that exosome membranes are enriched in the family of four transmembrane proteins involved in transport (CD63, CD8, and CD9) and the family of heat shock proteins (HSP60, HSP70, and HSP90). Using CD63 as a biomarker, by lentivirally coupling CD63 to fluorescent proteins, fluorescent proteins will be expressed on the exosome membrane, facilitating downstream functional analysis experiments.
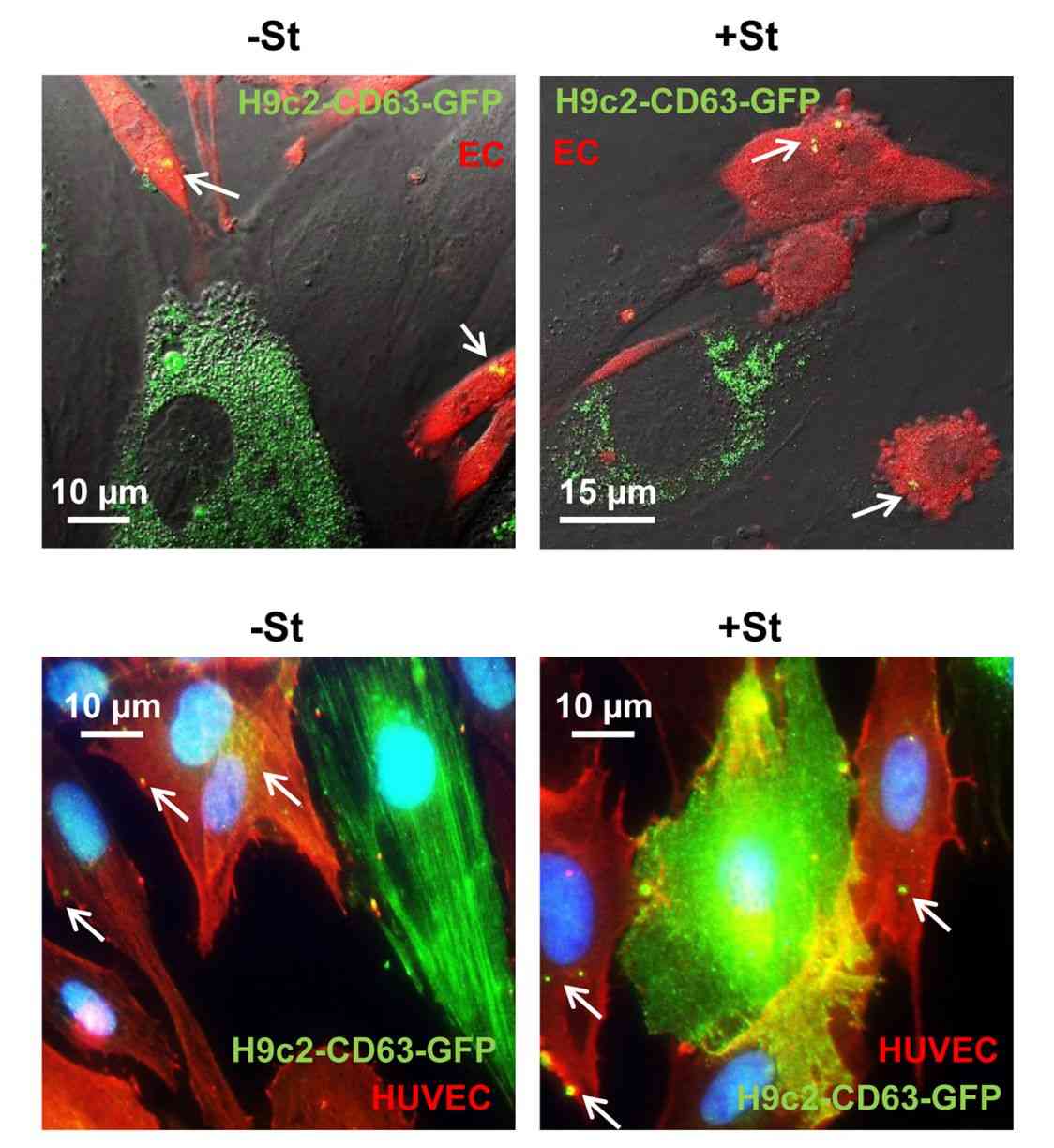 Figure 1. Transfection of H9C2 with CD63-GFP lentiviral vector. (Garcia NA, et al., 2015)
Figure 1. Transfection of H9C2 with CD63-GFP lentiviral vector. (Garcia NA, et al., 2015)
What is the Principle of Using Lentiviral Labeling Exosomes?
Exosome-labeled lentiviruses allow real-time monitoring of exosome biogenesis, uptake, and biodistribution by integrating traceable reporter genes. Quantitative reporter gene expression allows sensitive measurement of exosomes released by transduced cells. Fluorescent labeling visualizes exosomes. The principle involves lentiviral integration of CD63 reporter gene fusions into secreted exosomes. Innovative tools are provided to elucidate exosome function and explore diagnostic/therapeutic potential.
Why Labeling Exosomes by Lentivirus-Mediated CD63-GFP Expression?
- Lentivirus
Lentiviruses are widely used in cell and animal experiments because they randomly integrate target genes into genomic DNA after infecting cells, and thus can stably express target proteins for a long period, can infect almost all mammalian cells, and can also stably express them for a long period in many non-dividing cells. The expression elements of CD63, a specific protein of exosomes, and GFP, a green fluorescent protein, are constructed into a plasmid and then packaged into a lentivirus, which is subsequently used to infect cells so that the exosomes secreted by the cells are green fluorescent.
- Exosome Marker Proteins
Exosome marker proteins can be used to identify, observe, label, and purify exosomes. According to the Exosome Database 9769 proteins have been identified in exosomes from different organisms, cell types, and body fluids, of which CD9, CD63, CD81, Alix, Flotillin, Ceramide, and Tumor susceptibility gene 101 (TSG101) are the most commonly used exosomal protein markers. CD63, also known as lysosome-associated membrane protein 3 (LAMP3), belongs to the Transmembrane 4 superfamily and is abundantly present on the surface of extracellular vesicles, and has been associated with cell activation, adhesion, and mutation, as well as tumor invasion and metastasis. cd63 is one of the most commonly used exosome protein markers, and is thought to be a key player in the regulation of extracellular vesicle production and intracellular material sorting, which provides opportunities for novel drug delivery and targeting exosomes, and also serves as a potential marker for tumors (e.g., ovarian cancer and lung cancer). Researchers have used recombinant CD63 protein to develop multiple aptamers for non-invasive exosome detection. The versatility of CD63 targets holds new promise for therapeutic, diagnostic, and biomedical research.
- Fluorescent Protein
Exosomes are released outside the cell in the form of exocrine glands after the fusion of intracellular multivesicular bodies with the cell membrane. According to the mechanism of exosome generation, modification of exosome membrane can be realized by genetic engineering of parental cells. The fluorescent protein is a commonly used reporter protein that can be excited by specific wavelengths of excitation light and emit corresponding fluorescent signals. Parental cells can be genetically modified by infection with a viral vector inserting the fluorescent protein coding sequence and the exosomal membrane protein coding sequence. These modified cells then secrete exosomes with fluorescent proteins to track the exosomes from the donor to the recipient.
Application
- Exosome quantification
- Exosome biodistribution research
- Exosome animal research tracking
- Cellular uptake research
- Cell targeting research
- Library screening
Why Choose Lentivirus-Mediated CD63-GFP Expression to Label Exosomes?
- Real-time Tracking - Monitor exosome dynamics in real-time using an integrated reporter.
- Sensitive Measurement - Enables precise measurement of exosome release and biodistribution.
- Fluorescent Labeling - Enables exosome imaging and quantification through fluorescent labeling.
- Innovative Tools - Provides innovative tools to elucidate exosome function and its application in biomarkers and therapeutics.
Related Products
Creative Biostructure selects appropriate fluorescent dyes or customized for exosome labeling according to clients' requirements. After labeling, we provide observation methods such as fluorescence microscopy, flow cytometry, and confocal microscopy to research the distribution and dynamic changes of exosomes. In addition, we provide high-quality fluorescent exosome/microvesicle products to evaluate the role and function of exosomes.
| Cat No. | Product Name | Source |
| LEV-F665-LnCAP | HQExo™ Microvesicles-665-LnCAP | Fluorescent labeled microvesicles derived from human prostate adenocarcinoma (LnCAP cell line) |
| Exo-F488-A549 | HQExo™ Exosome-488-A549 | Fluorescent labeled exosome derived from human non-small cell lung cancer cell line (A549 cell line) |
| Exo-F488-B16F10 | HQExo™ Exosome-488-B16F10 | Fluorescent labeled exosome derived from murine melanoma cell line (B16F10 cell line) |
| Exo-F488-BLCL | HQExo™ Exosome-488-BLCL | Fluorescent labeled exosome derived from EBV transformed lymphoblastoid B cells (BLCL21 cell line) |
| LEV-F665-HEK293 | HQExo™ Microvesicles-665-HEK293 | Fluorescent labeled microvesicles derived from HEK293 cell line |
| LEV-F665-MM1 | HQExo™ Microvesicles-665-MM1 | Fluorescent labeled microvesicles derived from human melanoma (MM1 cell line) |
| Explore All Fluorescent Exosomes/Microvesicles | ||
Creative Biostructure insists on providing high-quality exosome labeling services with flexible methods and accurate results. We always work with our clients to solve specific challenges and pursue the highest level of client satisfaction. Feel free to contact us for more details and to find out how it can help your research.
References
- Garcia NA, et al. Glucose Starvation in Cardiomyocytes Enhances Exosome Secretion and Promotes Angiogenesis in Endothelial Cells. PLoS One. 2015. 10(9): e0138849.
- Ichikawa H, et al. Exosome Transfer Between Pancreatic-cancer Cells Is Associated With Metastasis in a Nude-mouse Model. Anticancer Res. 2021. 41(6): 2829-2834.
- Ren R, et al. Bone marrow mesenchymal stem cell-derived exosome uptake and retrograde transport can occur at peripheral nerve endings. Artif Cells Nanomed Biotechnol. 2019. 47(1): 2918-2929.
