Labeling Exosomes by Membrane-permeable Compound Tracers
Exosomes are of interest to the scientific community because of their role in physiological and pathological processes and the potential for therapeutic applications. They range from 30 nm to 150 nm and usually consist of a phospholipid bilayer containing biologically active molecules such as proteins and nucleic acids. Detecting and analyzing exosomes by flow cytometry has been challenging because the use of non-specific membrane dyes or coupled antibody staining against surface markers of specific cellular origins cannot distinguish between intact exosomes and fragments. In addition, the use of antibodies alone to detect exosomes limits research to a priori knowledge of the desired cell of origin. An emerging strategy for flow cytometry-based exosome analysis is labeling with membrane-permeable molecules.
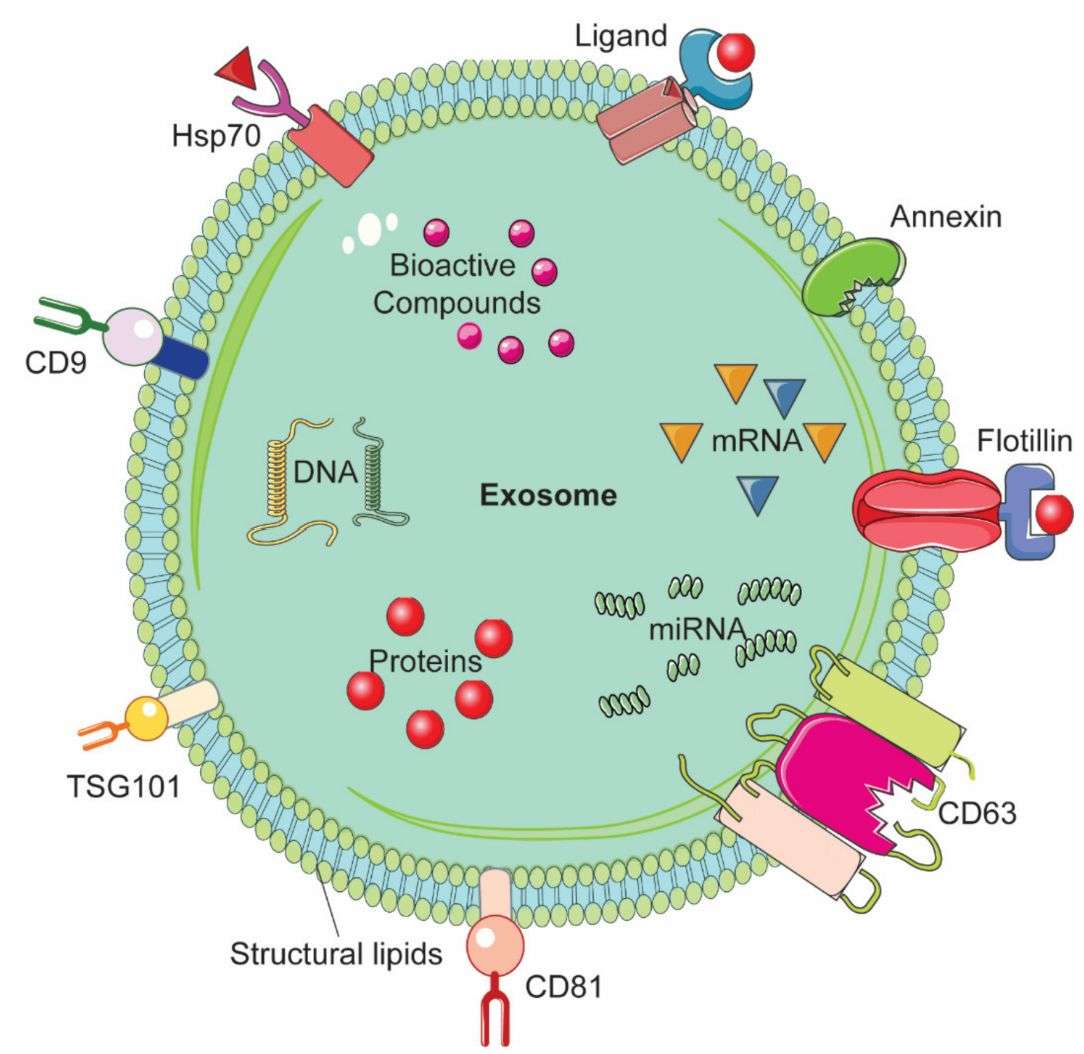 Figure 1. Representative structure of an exosome and its composition. (Gaurav I, et al., 2021)
Figure 1. Representative structure of an exosome and its composition. (Gaurav I, et al., 2021)
What is Membrane Permeable Compound Labeling?
Membrane-permeable compound labeling assays are a technique used in biological research to label or tag certain cells or cellular components using specific compounds that can easily cross cell membranes. These labeled compounds can then be detected and tracked, allowing researchers to monitor cellular processes, interactions, and reactions in vitro and in vivo. This approach is particularly useful in research involving exosome transport, migration, and drug delivery.
What are Membrane Permeable Compound Markers?
Membrane permeable compound markers are substances that can easily cross membranes due to their specific properties. They are commonly used in biological research to track, label, or characterize exosomes. These markers can provide essential data about the function and structure of exosomes, the distribution of specific molecules, and the response of exosomes to various stimuli. The use of membrane-permeable compound markers can greatly facilitate research in areas such as drug delivery and disease diagnosis. The commonly used membrane permeability markers are as follows.
- CFSE (carboxyfluorescein diacetate succinimidyl ester)
CFSE is a commonly used cell labeling reagent that is without color or fluorescence until an intracellular esterase enzyme removes its acetate group to produce the carboxyfluorescein succinimidyl ester that emits bright fluorescence. The succinimidyl ester reacts with intracellular amino groups to form a fluorescent coupling that is stable and can be immobilized with aldehyde fixatives. CFSE can passively diffuse into cells, readily cross exosome membranes, and covalently bind to intracellular proteins. Because of its simplicity and compatibility with various downstream applications, CFSE is widely used to track exosomes and research their uptake and translocation between cells.
- Calcein-AM
The introduction of an acetylmethoxymethyl ester (AM) moiety to conventional Calcein increases its hydrophobicity, allowing it to easily penetrate living cell membranes. Once inside the exosome, Calcein-AM (which does not fluoresce by itself) is sheared by esterases within the exosome to form the membrane-impermeable polar molecule Calcein, which is retained inside the cell and emits strong green fluorescence. It is commonly used to research exosome uptake processes.
Advances in Research on Labeling Exosomes with Membrane Permeable Compound Markers
- Amine-Reactive Dye CFSE as Exosome Labeling
Because lipid and RNA dyes produce artifacts, researchers tested the protein-binding dye 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE). CFSE alone did not form aggregates, and CFSE-stained exosomes maintained a light scattering pattern similar to unstained ones. In addition, nanoFACS event rates and NTA analysis of particle concentration and size distributions of CFSE-stained exosome samples were similar to those observed in unstained samples. Using spikes in the beads to analyze particle concentrations after each labeling method quantitatively, both nanoFACS and NTA data confirmed that CFSE does not undergo nonspecific aggregation compared to lipophilic dyes and RNA-binding dyes and is suitable for nanoscale flow cytometry staining of individual exosomes for analytical and quantitative purposes.
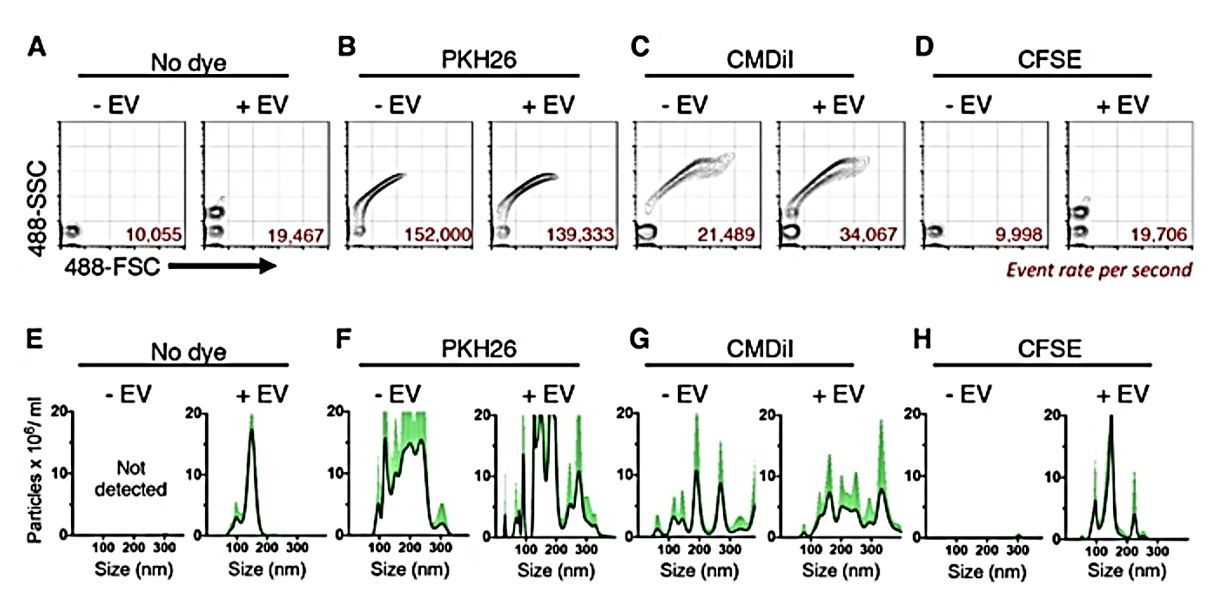 Figure 2. EV label suitability analysis by nanoFACS HR-FCM and NTA. (Morales-Kastresana A, et al., 2017)
Figure 2. EV label suitability analysis by nanoFACS HR-FCM and NTA. (Morales-Kastresana A, et al., 2017)
- Calcein-AM Suitable for Detecting Intact Exosomes by Flow Cytometry
Researchers describe a simple and highly sensitive, accurate, and precise flow cytometry-based method that uses calcein-AM to detect and quantify exosomes of various origins. In addition, the research demonstrates that the method can differentiate between intact and disrupted exosomes and is valuable in assessing vesicle stability, particularly the tolerance of exosomes to changes in temperature, freeze/thaw cycles, enzyme activity, extracellular environment, pH, osmolality, and time. Isolation of exosomes from multiple sources, followed by treatment with calcein-AM solution and incubation to allow exosomes to fluoresce calcein-AM by endoesterase activity. Data are then collected by flow cytometry. Incubation with 10 μM calcein-AM for 20 minutes is found to be the standard condition to achieve maximum fluorescence of the different exosomes.
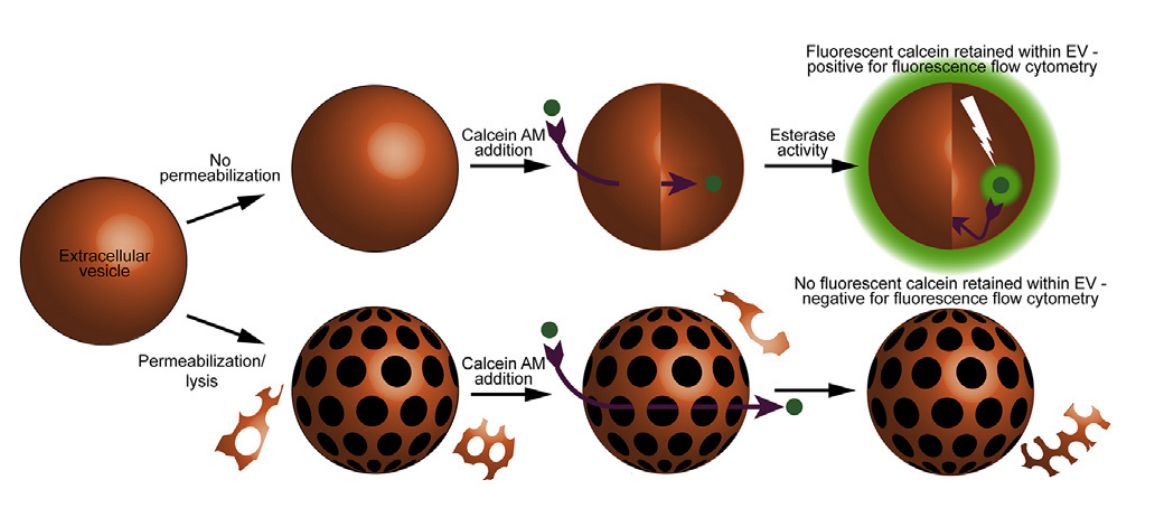 Figure 3. Labeling exosomes with calcein-AM. (Gray WD, et al., 2015)
Figure 3. Labeling exosomes with calcein-AM. (Gray WD, et al., 2015)
Why Choose Membrane-permeable Compound Tracers to Label Exosomes?
- Easy to Use - Membrane-permeable compound tracers are generally easy to use and can be added directly to exosome-containing samples for simple and effective labeling.
- Versatile Labeling - Membrane-permeable compound tracers can label a wide range of exosomes without the need for specific surface markers or genetic modifications.
- Homogeneous Labeling - These tracers diffuse into the exosome and are evenly distributed throughout the exosome, resulting in homogeneous labeling.
- Minimal Perturbation of Exosome Biology - Membrane-permeable compound tracers typically have minimal impact on exosome biology and function. They do not require genetic modification or alteration of exosome characteristics.
Our Products
In addition to our membrane-permeable compound-based labeling exosome service, we offer a range of high-quality exosome products to help clients explore the potential applications of exosomes in the diagnosis and treatment of cancer and chronic diseases.
| Cat No. | Product Name | Source |
| Exo-CH06 | HQExo™ Exosome-MCF-7 | Exosome derived from human breast cancer, noninvasive cell line (MCF-7 cell line) |
| Exo-CH12 | HQExo™ Exosome-MM1 | Exosome derived from human melanoma (MM1 cell line) |
| Exo-CH18 | HQExo™ Exosome-LnCAP | Exosome derived from human prostate adenocarcinoma (LnCAP cell line) |
| Exo-GC03 | HQExo™ Exosome-GFP | Exosome derived from HEK293 cell line with GFP loaded |
| Exo-GC07 | HQExo™ Exosome-CD63-EGFP | Exosome derived from human embryonic kidney cell line (HEK293, CD63-EGFP) |
| Exo-IC01 | HQExo™ Exosome-BC3 | Exosome derived from human B lymphocyte cell line (BC-3 ) |
| Exo-SC02-2 | HQExo™ Exosome-Pla-MSC | Exosome derived from human placental derived mesenchymal stem cell |
| Explore All Exosomes Products | ||
Creative Biostructure is a leader in the field of exosome research, we provide our clients with a one-stop service from exosome isolation, characterization, labeling, tracing, targeting, and functional analysis to provide new insights into the behavior, interactions, and functions of exosomes. Please feel free to contact us for a formal quote.
References
- Gaurav I, et al. Factors Affecting Extracellular Vesicles Based Drug Delivery Systems. Molecules. 2021. 26(6): 1544.
- Morales-Kastresana A, et al. Labeling Extracellular Vesicles for Nanoscale Flow Cytometry. Sci Rep. 2017. 7(1): 1878.
- Gray WD, et al. An accurate, precise method for general labeling of extracellular vesicles. MethodsX. 2015. 2: 360-367.