Mempro™ Liposome Composition
Creative Biostructure has been devoted to developing liposome technology for years. We now have multiple types of liposome products available, and provide customized liposome production service to meet your requirements.
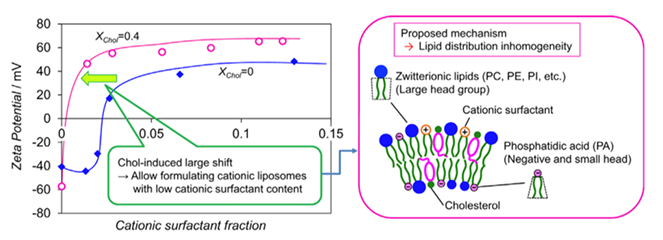 Figure 1. Schematic diagram of the membrane structure in liposomes (XChol > 0.4). The negatively-charged PA molecules are preferred to reside in the inner layer for its large CPP. (Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016)
Figure 1. Schematic diagram of the membrane structure in liposomes (XChol > 0.4). The negatively-charged PA molecules are preferred to reside in the inner layer for its large CPP. (Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016)
Liposomes can be prepared from a variety of lipids or their mixtures. Phosphatidyl choline (PC) is the predominant phospholipids found in natural membranes. The permanent positive charge on the choline of the headgroup counteracts the negative charge on the phosphate, which gives a neutral, very hydrophilic headgroup. In a membrane, interaction between the tertiary ammonium group and phosphates on adjacent molecules can contribute to the tightness of packing and help to disperse local fluctions in charge density.
Sphingomyelin (SM) has a more highly ordered gel phase than phosphatidyl choline, which can be explained by the hydrogen bond interactions permitted by the amide linkage and hydroxyl groups in SM. Membrane packing of SM is tighter than it of PC. The highly-ordered structure of SM results in a lower permeability to solutes, greater resistance to lysis by bile salts, and reduction of membrane fluidity.
Negatively charged phospholipids such as phosphatidyl serine (PS) can bind strongly to cations, particularly divalent cations such as magnesium and calcium. This binding reduces the electrostatic charge on the molecule. Moreover, a high proportion of negatively charged phospholipids may cause the membrane to condense. The reduction in electrostatic repulsion also can result in the aggregation of liposomes. Absence of any substitution on the phosphate in phosphatidic acid (PA) confers a very strong negative charge to the molecule. Dispersions of PA alone in water have a pH of between 2 and 3, and rapid neutralization with acid can cause membrane reorganization, under the influence of electrostatic effects, to produce unilamellar vesicles.
Phosphatidyl glycerol (PG) shows a permanent negative charge under the normal physiological pH range. PG can be isolated directly from natural sources, and it also can be semi-synthetically prepared from other lipids by using phospholipase D in the presence of glycerol.
Phosphatidyl ethanolamine (PE) has a transition temperature approximately 20°C higher than their PC analogues. At low pH, the PE molecules become more protonated, reducing formation of hydrogen bonds and resulting in transition temperatures similar to PC. Because of charge neutralization, liposomes with high levels of PE tend to aggregate in the presence of Ca2+ and Mg2+ ions.
Depended on the different composition, properties of liposomes can vary quite a lot. Creative Biostructure is your reliable scientific partner to develop and produce custom liposomes. Please feel free to contact us for a detailed quote.
References:
I. Ahmad, et al. (2016). Formulation and stabilization of norfloxacin in liposomal preparations. European Journal of Pharmaceutical Sciences, 91: 208-215.
R. G. Brea, et al. (2016). Nonenzymatic biomimetic remodeling of phospholipids in synthetic liposomes. PNAS, 113(31): 8589-8594.
K. Aramaki, et al. (2016). Charge boosting effect of cholesterol on cationic liposomes. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 506: 732-738.