Eukaryotic Flagella/Cilia Structure
Eukaryotic flagella and cilia are essential cellular appendages that play critical roles in motility, signaling, and sensory functions in a wide variety of organisms. Despite their different names, these structures are remarkably similar in their basic architecture and differ primarily in their size and mode of movement. Understanding the structure and function of eukaryotic flagella and cilia is not only key to understanding how cells move and interact with their environment, but also provides insight into many human diseases caused by defects in these organelles, known as ciliopathies.
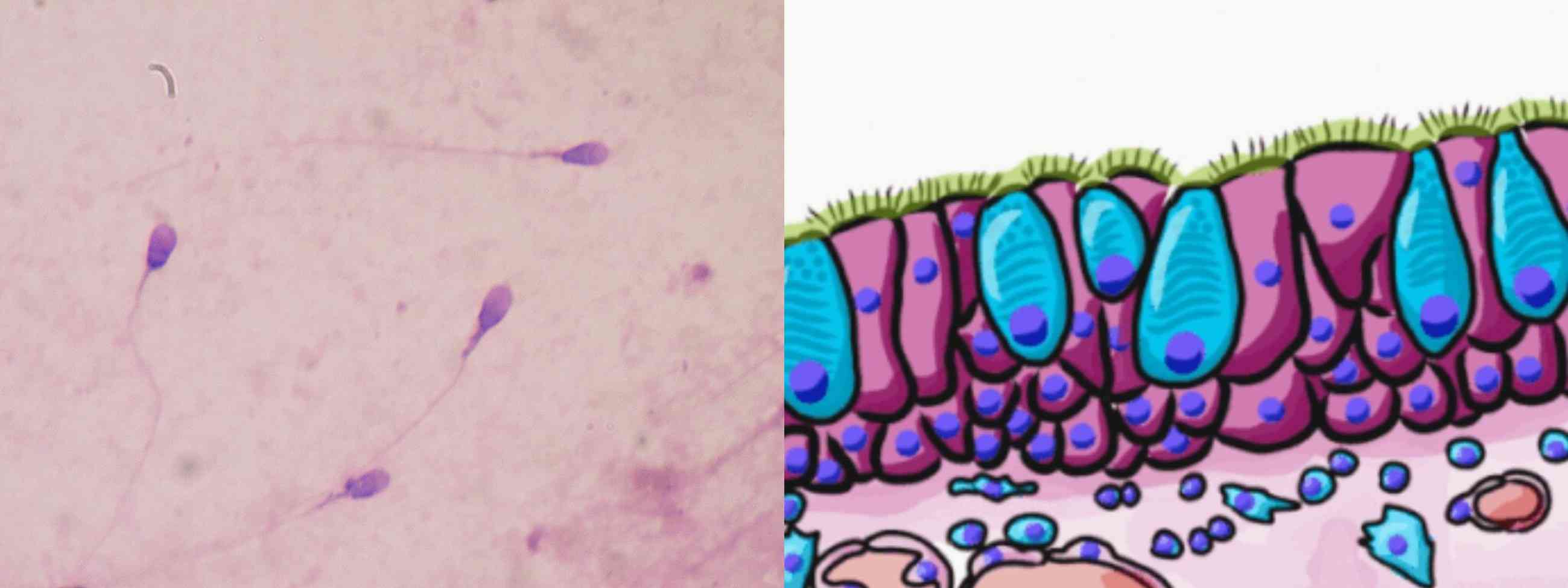 Figure 1. Example of eukaryotic flagella (left, human sperm cells) and cilia (right, human pseudostratified columnar epithelium).
Figure 1. Example of eukaryotic flagella (left, human sperm cells) and cilia (right, human pseudostratified columnar epithelium).
What Are the Eukaryotic Flagella and Cilia?
Eukaryotic flagella and cilia are complex, hair-like structures that extend from the surface of many eukaryotic cells. They are primarily involved in cell movement, either moving the entire cell (as seen in sperm cells) or moving fluid across the cell surface (as seen in the epithelial cells lining the respiratory tract). Although the terms "flagella" and "cilia" are often used interchangeably, they differ in length and pattern of movement. Flagella are usually longer, often found in smaller numbers, and have a wave-like motion that propels the cell forward. Cilia, on the other hand, are typically shorter, more numerous, and move in a coordinated back and forth motion known as an action stroke followed by a recovery stroke. The differences between cilia and flagella are summarized below.
| Characteristics | Flagella | Cilia |
| Number | Comparatively less (usually ranges from 1 to 8). | Comparatively more (typically ranges in the thousands). |
| Length | Comparatively longer in length. | Usually shorter in length. |
| Occurrence | Presence at one end or two ends or all over the surface. | Occurs throughout the cell surface. |
| Motion | The beating pattern of flagella involves circular, wave-like or propeller-like motion. | The beating pattern of cilia is very complicated – It can move in a wide range of motions. |
| Found in | Found in prokaryotic cells and eukaryotic cells. | Found in eukaryotic cells. |
| Types | Three types: bacterial flagella, archaeal flagella and eukaryotic flagella. | Two types: non-motile cilia and motile cilia. |
| Energy Production | Powered by the proton-motive force by the plasma membrane. | Use "kinesin" which has an ATPase activity that produces energy to perform the movement. |
| Functions | Help mainly in locomotion only. | Help in locomotion, feeding circulation, aeration, etc. |
Structure of Eukaryotic Flagella and Cilia
The core of both flagella and motile cilia is a highly organized microtubule-based structure known as the axoneme. The axoneme is characterized by a "9+2" arrangement of microtubules, consisting of nine peripheral microtubule doublets surrounding a central pair of single microtubules. This structure is surrounded by the cell membrane, effectively making the flagellum or motile cilium an extension of the cell.
Axoneme
The microtubules that make up the axoneme are composed of tubulin, a globular protein that polymerizes to form the cylindrical structure of the microtubules. Each of the nine peripheral doublets consists of an "A" microtubule, which is a complete microtubule with 13 protofilaments, and a "B" microtubule, which is an incomplete microtubule with 10 or 11 protofilaments. The central pair of microtubules are complete, each with 13 protofilaments.
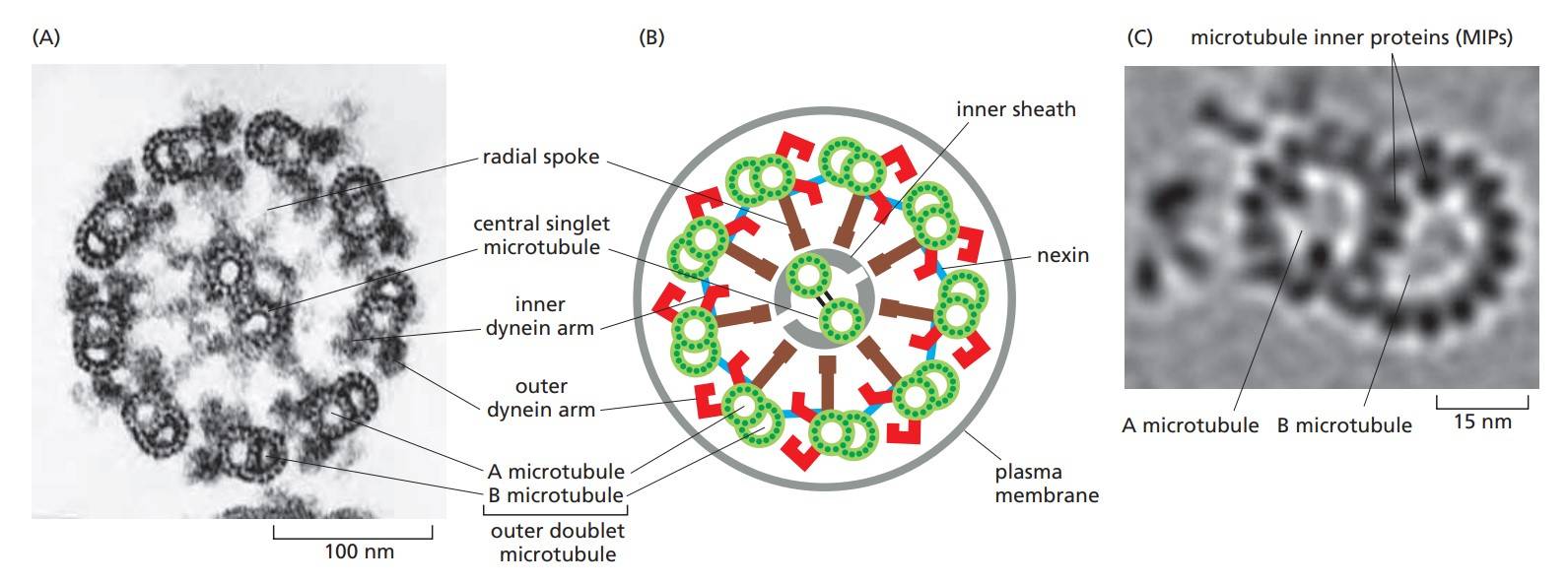 Figure 2. The arrangement of microtubules in a flagellum or cilium. (A) Electron micrograph of the flagellum of a green-alga cell (Chlamydomonas) shown in cross section. (B) Diagram of the parts of a flagellum or cilium. (C) High-resolution electron tomography image of an outer doublet microtubule showing structural details and features inside the microtubules called microtubule inner proteins (MIPs) (Molecular Cell Biology of the Cell, 6th Edition).
Figure 2. The arrangement of microtubules in a flagellum or cilium. (A) Electron micrograph of the flagellum of a green-alga cell (Chlamydomonas) shown in cross section. (B) Diagram of the parts of a flagellum or cilium. (C) High-resolution electron tomography image of an outer doublet microtubule showing structural details and features inside the microtubules called microtubule inner proteins (MIPs) (Molecular Cell Biology of the Cell, 6th Edition).
Interspersed among the microtubules are several accessory proteins that are critical for the stability and function of the axoneme. The most notable of these are the dynein arms, which are motor proteins that generate the force required for the bending motions of the flagella and cilia. Dynein arms are attached to the "A" microtubule of each doublet and extend toward the "B" microtubule of the adjacent doublet. These arms use ATP to "walk" along the adjacent microtubule, causing the doublets to slide against each other. This sliding motion, when constrained by other proteins such as nexin links and radial spokes, results in bending of the axoneme, leading to the characteristic beating pattern of cilia and flagella.
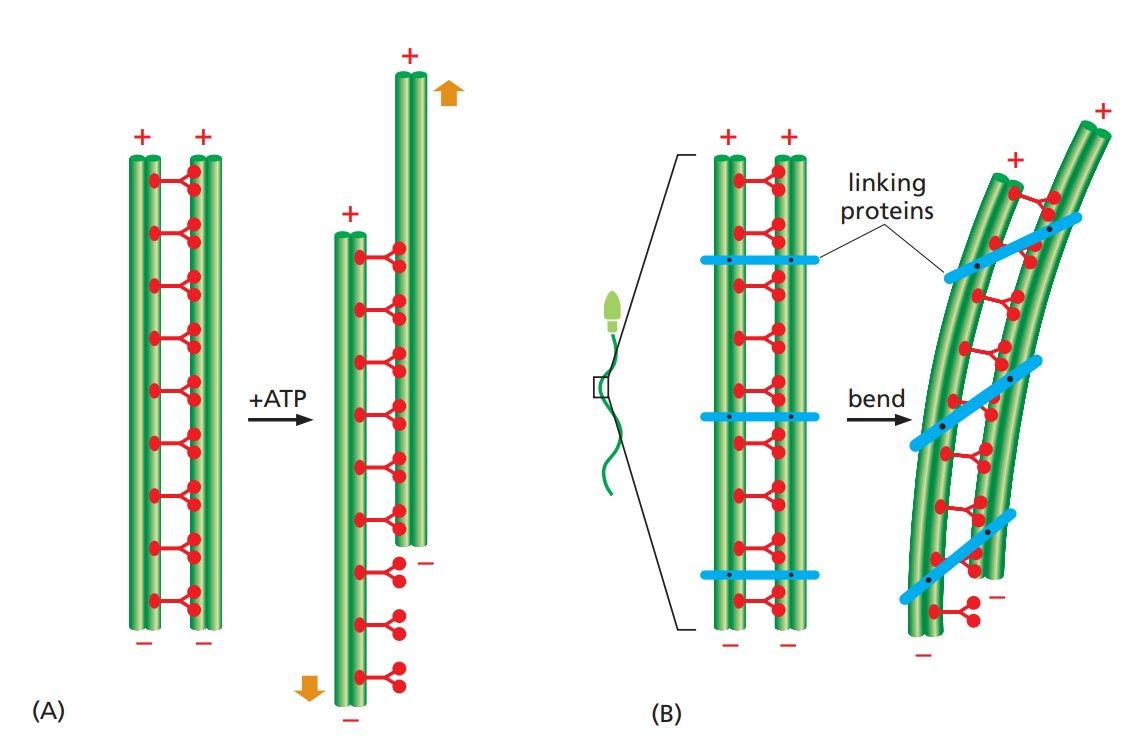 Figure 3. The bending of an axoneme. (A) When axonemes are exposed to the proteolytic enzyme trypsin, the linkages holding neighboring doublet microtubules together are broken. In this case, the addition of ATP allows the motor action of the dynein heads to slide one pair of doublet microtubules against the other pair. (B) In an intact axoneme (such as in a sperm), flexible protein links prevent the sliding of the doublet. The motor action therefore causes a bending motion, creating waves or beating motions (Molecular Cell Biology of the Cell, 6th Edition).
Figure 3. The bending of an axoneme. (A) When axonemes are exposed to the proteolytic enzyme trypsin, the linkages holding neighboring doublet microtubules together are broken. In this case, the addition of ATP allows the motor action of the dynein heads to slide one pair of doublet microtubules against the other pair. (B) In an intact axoneme (such as in a sperm), flexible protein links prevent the sliding of the doublet. The motor action therefore causes a bending motion, creating waves or beating motions (Molecular Cell Biology of the Cell, 6th Edition).
Basal Body and Transition Zone
The axoneme is anchored to the cell at the base of the flagellum or cilium by the basal body, which is structurally similar to the centriole found in the centrosome of the cell. The basal body consists of a cylindrical array of nine triplet microtubules and serves as a template for the assembly of the axoneme. The basal body is also involved in the docking of the flagellum or cilium to the plasma membrane and plays a critical role in the initiation and regulation of axoneme assembly.
Between the basal body and the axoneme lies the transition zone, a region where the triplet microtubules of the basal body gradually transition into the doublet microtubules of the axoneme. The transition zone serves as a gatekeeper, regulating the entry and exit of proteins and other molecules into the flagellum or cilium. This is crucial for maintaining the specialized environment of the cilium or flagellum and ensuring proper assembly and function.
Non-Motile Cilia
Remarkably, unlike motile cilia, non-motile cilia typically have a "9+0" microtubule arrangement, meaning they have nine peripheral microtubule doublets but lack the central microtubule pair found in motile cilia. They do not have dynein arms because they are not involved in generating movement. Their function is primarily sensory or signaling rather than motile. Functionally, non-motile cilia, often referred to as primary cilia, act as sensory organelles that detect mechanical, chemical, and light signals. They play critical roles in signaling pathways, including the Hedgehog signaling pathway, which is essential for several developmental processes.
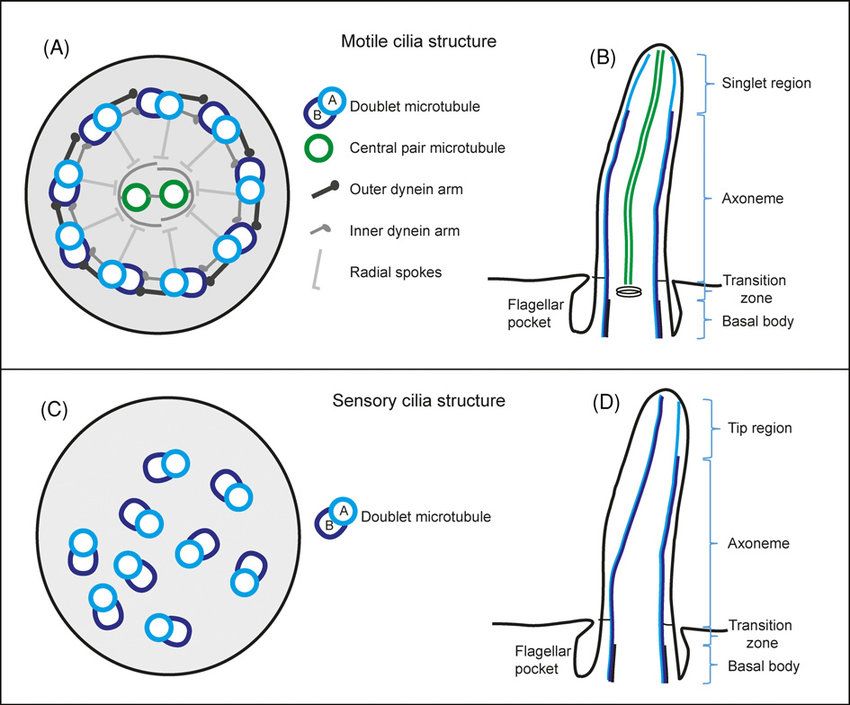 Figure 4. Schematic drawing of eukaryotic motile and sensory cilia ultrastructure (Croft et al., 2018).
Figure 4. Schematic drawing of eukaryotic motile and sensory cilia ultrastructure (Croft et al., 2018).
The Role of Intraflagellar Transport
Intraflagellar transport (IFT) is a bidirectional, motor-driven process that is essential for the assembly, maintenance, and function of cilia and flagella. IFT involves the movement of large protein complexes, known as IFT particles, along the microtubules of the axoneme. Kinesin motor proteins drive anterograde transport, moving IFT particles from the cell body toward the tip of the cilium or flagellum, while cytoplasmic dynein motors drive retrograde transport, moving IFT particles back toward the cell body. IFT is responsible for delivering the building blocks necessary for axonemal assembly, as well as for removing damaged or obsolete components.
Methods for Studying Eukaryotic Flagella and Cilia
Studying the structure and function of eukaryotic flagella and cilia requires a combination of advanced imaging techniques, molecular biology, and biochemical approaches.
| Techniques | Description |
| Electron Microscopy | Electron microscopy (EM) has been indispensable in revealing the intricate details of axoneme structure. Transmission electron microscopy (TEM) allows high-resolution imaging of thin sections of flagella or cilia, revealing the "9+2" arrangement of microtubules, dynein arms, radial spokes, and other structural components. Scanning electron microscopy (SEM), on the other hand, provides detailed images of the cell surface, allowing researchers to observe the overall morphology and distribution of cilia and flagella on the cell surface. |
| Fluorescence Microscopy | Fluorescence microscopy, particularly super-resolution techniques like stimulated emission depletion (STED) microscopy and structured illumination microscopy (SIM), has become a powerful tool for studying the dynamic processes within cilia and flagella. By tagging specific proteins with fluorescent markers, researchers can track the movement of IFT particles, dynein, and other key components in real time. This approach has been instrumental in understanding how these structures are assembled, how they function, and how their dysfunction leads to disease. |
| Cryo-Electron Tomography | Cryo-electron tomography (cryo-ET) is an advanced technique that combines the high-resolution capabilities of EM with three-dimensional imaging. In cryo-ET, samples are rapidly frozen to preserve their native structure and then imaged from multiple angles to create a 3D reconstruction. This method has provided unprecedented insight into the architecture of the axoneme and the arrangement of molecular complexes within cilia and flagella. |
| Genetic and Molecular Approaches | Genetic manipulations such as RNA interference (RNAi), CRISPR-Cas9 gene editing, and transgenic animal models have allowed researchers to dissect the molecular mechanisms underlying cilia and flagellar function. By selectively knocking out or modifying specific genes, scientists can study the effects on cilia and flagellar assembly, motility, and signaling. These approaches are particularly useful for identifying the role of individual proteins in ciliogenesis and for modeling human ciliopathies in animal systems. |
| Biochemical Techniques | Biochemical methods, such as protein purification, mass spectrometry, and co-immunoprecipitation, are used to identify and characterize the proteins that make up the cilia and flagella. These techniques can provide information about the composition of the axoneme, the interactions between different structural proteins, and the regulatory mechanisms that control cilia and flagellar function. |
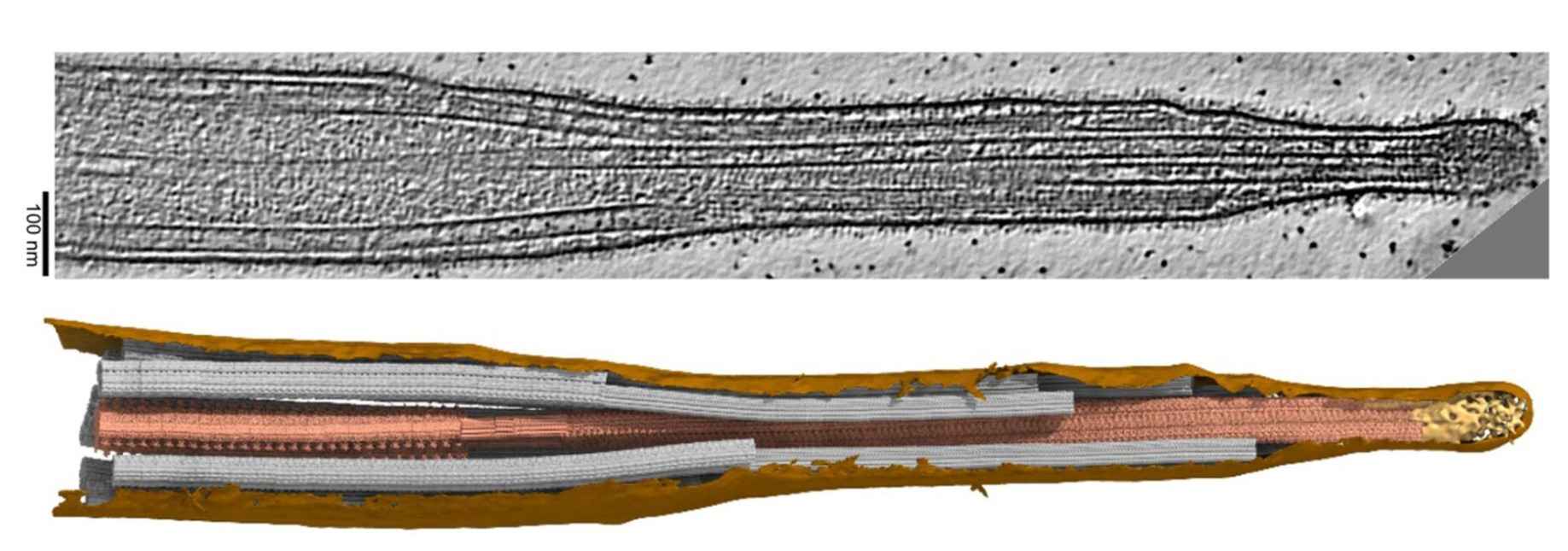 Figure 5. Example of ciliary structure studied by cryo-electron tomography. Membrane: brown, central pair: dark salmon, doublets and singlet A-tubules: grey, cap complex: gold (Legal et al., 2023).
Figure 5. Example of ciliary structure studied by cryo-electron tomography. Membrane: brown, central pair: dark salmon, doublets and singlet A-tubules: grey, cap complex: gold (Legal et al., 2023).
Eukaryotic flagella and cilia are highly specialized and complex organelles that are essential for a wide range of cellular functions, from motility to sensory perception and signal transduction. The intricate structure of the axoneme, the coordinated action of motor proteins, and the precise regulation of protein trafficking are all key to the proper function of these organelles.
Creative Biostructure is dedicated to the detailed analysis of subcellular structures. We offer comprehensive assay services for the study of eukaryotic flagella/cilia structures, which have provided profound insights into the structure and function of cilia and flagella and have shed light on the molecular basis of ciliopathies, to support your research into the understanding and treatment of diseases associated with defects in these critical cellular structures. To explore how our services can benefit your research, please contact us for a personalized solution.
References
- Molecular Biology of the Cell, 6th edition.
- Croft, J. T., Zabeo, D., Subramanian, R., & Höög, J. L. (2018). Composition, structure and function of the eukaryotic flagellum distal tip. Essays in Biochemistry, 62(6), 815–828.
- Legal, T., Tong, M., Black, C., Valente Paterno, M., Gaertig, J., & Bui, K. H. (2023). Molecular architecture of the ciliary tip revealed by cryo-electron tomography. bioRxiv, 2023.01.03.522627.