Structural Research of Betaine/Choline/Carnitine Transporter (BCCT) Family
The growth of microorganisms is a multifaceted process that is heavily influenced by changes in environmental osmolarity. It is paramount for these microorganisms to maintain a physiologically acceptable level of cellular hydration and turgor when faced with high osmolarity, which is achieved through the accumulation of compatible solutes. These compounds are often scavenged from scarce environmental sources using osmotically controlled uptake systems, which are highly effective osmoprotectants. The betaine/choline/carnitine/transporter (BCCT) family is responsible for a number of these osmotically controlled uptake systems. These systems include sodium- or proton-coupled transporters such as BetP and BetT, which are ubiquitous in microorganisms. The BCCT family also contains CaiT, an l-carnitine/γ-butyrobetaine antiporter that is not directly involved in osmotic stress responses.
A representative of the osmoregulated symporters in the BCCT family is the glycine betaine transporter BetP from Corynebacterium glutamicum. BetP functions as both an osmosensor and osmoregulator, making it an integral component of the microorganism's response to osmotic stress. Using X-ray crystallography and lipidomics, the researchers provide evidence for specific lipid-binding sites within the BetP protein that are critical for its function. The study suggests that the lipid interactions with BetP are essential for the protein's stability, activity, and substrate specificity. The findings may have implications for the development of new drugs targeting bacterial transporters and provide insight into the molecular mechanisms of osmoregulation in bacteria.
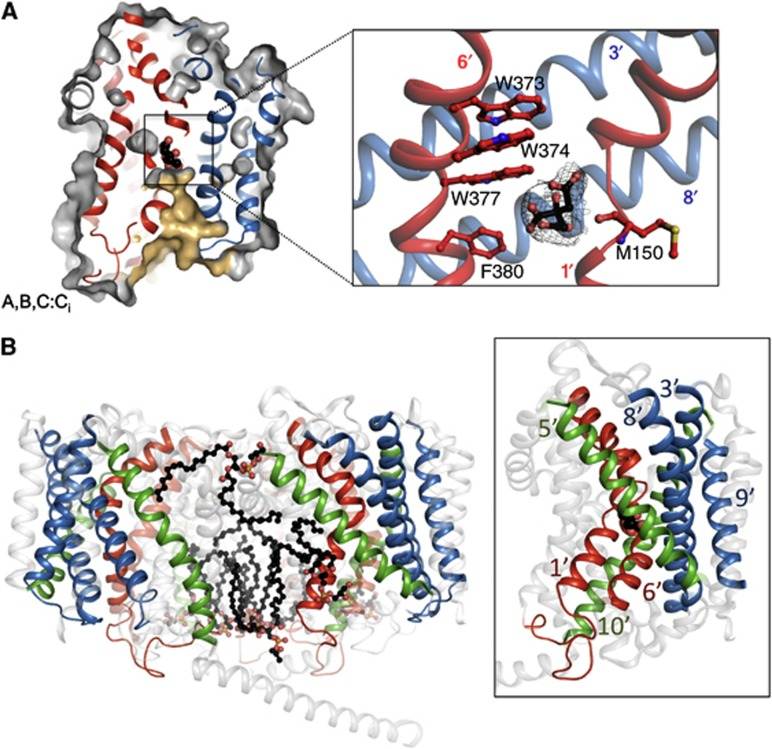 Figure 1. Inward-open state of BetP. (Koshy C, et al., 2013)
Figure 1. Inward-open state of BetP. (Koshy C, et al., 2013)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| BetP glycine betaine transporter (expressed in E. coli) | Corynebacterium glutamicum | X-ray diffraction | 3.35 Å | 2WIT |
| BetP glycine betaine transporter in outward-facing conformation (expressed in E. coli) | Corynebacterium glutamicum | X-ray diffraction | 3.25 Å | 4DOJ |
| BetP glycine betaine transporter with bound lipids (expressed in E. coli) | Corynebacterium glutamicum | X-ray diffraction | 2.70 Å | 4C7R |
| CaiT carnitine transporter (expressed in E. coli) | Escherichia coli | X-ray diffraction | 3.15 Å | 3HFX |
| CaiT carnitine transporter | Escherichia coli | X-ray diffraction | 3.50 Å | 2WSX |
| CaiT carnitine transporter | Proteus mirabilis | X-ray diffraction | 2.29 Å | 2WSW |
| CaiT R262E bound to gamma-butyrobetaine (expressed in E. coli) | Proteus mirabilis | X-ray diffraction | 3.29 Å | 4M8J |
Table 1. Structural Research of Betaine/Choline/Carnitine Transporter (BCCT) Family.
At Creative Biostructure, we offer advanced protein structure analysis services to support research on the BCCT family and other transporters. Our services include X-ray crystallography, cryo-electron microscopy (Cryo-EM), nuclear magnetic resonance (NMR) spectroscopy, and computational modeling. We work closely with our clients to provide tailored solutions that meet their research needs.
Our experienced team of structural biologists and biochemists uses state-of-the-art equipment and software to produce high-quality protein structures. For example, our Cryo-EM service provides resolution down to the atomic level, making it an excellent tool for characterizing large membrane proteins. Our protein crystallization service is also highly effective and has produced structures for numerous membrane transporters and other proteins. If you are interested in working with us to study the structure of the BCCT family or other membrane transporters, please contact us today.
References
- Kalayil S, Schulze S, Kühlbrandt W. Arginine oscillation explains Na+ independence in the substrate/product antiporter CaiT. Proceedings of the National Academy of Sciences. 2013, 110(43): 17296-17301.
- Koshy C, et al. Structural evidence for functional lipid interactions in the betaine transporter BetP. The EMBO Journal. 2013, 32(23): 3096-3105.
- Perez C, et al. Alternating-access mechanism in conformationally asymmetric trimers of the betaine transporter BetP. Nature. 2012, 490(7418): 126-130.