Cryo-electron microscopy (cryo-EM) has become one of the most powerful techniques in structural biology for visualizing macromolecules at the atomic level. As cryo-EM technology advances, the integration of artificial intelligence (AI) has started to revolutionize data analysis, significantly enhancing accuracy, efficiency, and resolution. In this article, we explore the pivotal role AI plays in cryo-EM data analysis, from particle picking to 3D reconstruction and beyond, and how it is accelerating breakthroughs in drug discovery and structural biology.
Introduction to Cryo-EM and AI Integration
What is Cryo-EM?
Cryo-EM is a cutting-edge imaging technique used to determine the structures of biomolecules at near-atomic resolution by observing them in a frozen state. This technique has gained prominence for its ability to study large and complex biological structures that are challenging to analyze using traditional X-ray crystallography or nuclear magnetic resonance (NMR) spectroscopy.
The Role of AI in Cryo-EM Data Analysis
Artificial intelligence, particularly machine learning and deep learning, has transformed various aspects of cryo-EM data processing. By automating time-consuming and computationally intensive tasks, AI is improving the accuracy of particle detection, enhancing 3D reconstructions, and providing more reliable insights into the structures of biomolecules.
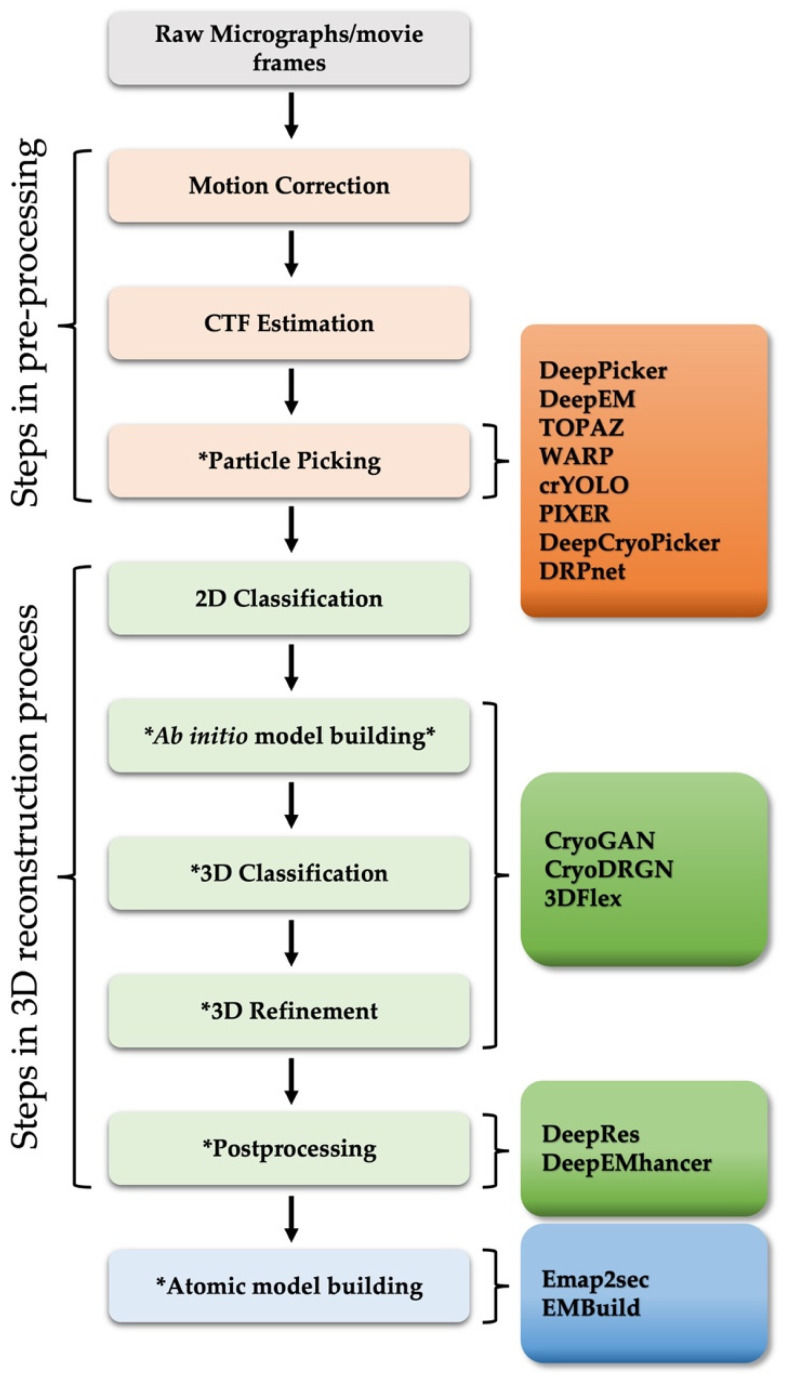 Figure 1. General Workflow for Single-Particle Analysis (SPA) 3D Reconstruction. This diagram outlines the SPA 3D reconstruction workflow, highlighting deep learning applications. (Chung J M, et al., 2022)
Figure 1. General Workflow for Single-Particle Analysis (SPA) 3D Reconstruction. This diagram outlines the SPA 3D reconstruction workflow, highlighting deep learning applications. (Chung J M, et al., 2022)
Applications of AI in Cryo-EM Data Analysis
Particle Picking and Image Processing
Particle picking is a critical step in cryo-EM data processing, where individual particles are identified and extracted from raw micrographs. Traditional methods, while effective to a degree, face several limitations that AI-based approaches can address.
Limitations of Traditional Particle Picking Methods
Traditional particle picking methods often rely on manual intervention and template-based approaches to detect particles in cryo-EM micrographs. These methods can be particularly inefficient when dealing with low signal-to-noise ratio (SNR) data (SNR < 0.1) or particles with irregular shapes, such as membrane proteins. The process can also be prone to errors, as it is highly dependent on the user's skill and the quality of the initial dataset.
AI Solutions for Particle Picking
AI-powered tools have revolutionized the particle picking process, addressing the limitations of traditional methods. By leveraging deep learning and neural networks, AI tools can automate particle selection with much higher accuracy, even in noisy datasets or with complex particle shapes.
| AI Tools | Description |
|---|---|
| SuperCryoEMPicker | One of the classical AI methods for particle picking. For challenging datasets, such as those containing β-galactosidase or other complex samples, SuperCryoEMPicker employs a superpixel clustering algorithm to enhance its robustness against noise. This method significantly improves the detection of particles in noisy environments, making it ideal for datasets with low SNR or irregular particle shapes. |
| DeepPicker | One of the leading AI-based particle picking tools, DeepPicker, uses a dual-module convolutional neural network (CNN) to automatically detect candidate particles. The tool relies on pre-trained models and has demonstrated performance close to human-level accuracy, significantly improving efficiency and accuracy in particle detection. |
| DeepEM | DeepEM is an AI-based tool that applies a deep learning framework to automate particle detection, improving sensitivity and accuracy, particularly for low SNR data. |
| DRPnet | DRPnet employs a regression network to directly predict particle locations, reducing the need for computationally expensive sliding window techniques. This approach allows for faster and more efficient particle detection, particularly in large-scale datasets, while maintaining high accuracy in particle localization. |
| TOPAZ, WARP, and crYOLO | AI-powered particle-picking tools that are gaining widespread adoption. These tools leverage CNNs and object detection techniques to automate particle selection efficiently, even from datasets with low SNR. |
By integrating these AI-driven solutions, researchers can not only speed up the particle picking process but also achieve higher quality and more reproducible results. AI-based tools are particularly effective at handling noisy, low-resolution, or complex datasets, which are often the norm in cryo-EM experiments.
3D Reconstruction and Refinement
In cryo-EM, 3D reconstruction is the process of converting 2D images into a 3D map of the macromolecule. Traditionally, this process is computationally intensive, requiring accurate pose estimation of each particle. AI has enhanced this process by improving pose estimation and reducing model bias, which often arises from traditional methods. AI-driven approaches, such as those utilizing stochastic gradient descent (SGD) or deep learning models, can refine 3D reconstructions more efficiently by providing better initial model predictions.
Popular software packages like CryoSPIN and SPIN incorporate AI techniques for refining 3D maps, resulting in higher resolution and improved structural insights. AI tools also help improve the alignment of individual particles during the reconstruction phase, contributing to more accurate 3D volumes.
Traditional Bottlenecks in Cryo-EM 3D Reconstruction
In traditional cryo-EM workflows, one of the most significant challenges in 3D reconstruction is determining particle orientations and merging the 2D projections into a 3D model. With datasets that may contain hundreds of thousands of particles (such as 200,000 particles), this process demands substantial computational resources and processing power. Researchers typically rely on high-performance computing clusters to manage and analyze these large datasets.
Additionally, the accuracy of 3D reconstruction can be limited by the initial particle orientations, which, if not correctly determined, can lead to inaccuracies in the final structure. The computational cost and time required for processing such vast amounts of data remain one of the primary bottlenecks in traditional cryo-EM workflows.
AI Optimization in Cryo-EM 3D Reconstruction
AI techniques have significantly advanced 3D reconstruction and refinement, enhancing both speed and accuracy. Here are some ways AI is improving this critical step:
| AI Tools | Description |
|---|---|
| Stochastic Gradient Descent (SGD) | SGD is a machine learning optimization technique commonly used in cryo-EM refinement to efficiently minimize the error in 3D reconstructions, speeding up the process of model fitting. |
| SPIN | SPIN (Structure Prediction and Iterative Refinement) integrates deep learning models with traditional cryo-EM refinement techniques, helping to predict structural features with greater precision and accelerating the refinement process. |
| CryoSPIN | CryoSPIN utilizes AI-driven algorithms to enhance cryo-EM data alignment and refinement, providing higher resolution and improving the accuracy of structural models. |
| AlphaFold-CryoEM Integration | AlphaFold, the AI-powered protein structure prediction tool, is now being integrated into cryo-EM workflows to accelerate the fitting of initial models into cryo-EM density maps. Using AlphaFold-generated models as initial templates helps guide the refinement process, ensuring better-fitting and higher-resolution structures. This is particularly beneficial for complex regions, such as transmembrane domains in proteins like the HIV SERINC3 protein, where fitting traditional models can be difficult. |
By integrating AI solutions, researchers can refine their 3D models more accurately and quickly. These advances not only improve the quality of cryo-EM data but also reduce the time required to achieve high-resolution structures, making the overall cryo-EM process more efficient.
Structural Heterogeneity Analysis
Structural heterogeneity is a fundamental aspect of many macromolecules, especially in systems like membrane proteins or multi-subunit complexes. The ability to analyze this heterogeneity is crucial for understanding functional dynamics and conformational changes in response to environmental or biochemical factors. Traditional methods have made strides in addressing this issue, but AI-driven solutions are now enhancing the ability to capture and differentiate subtle structural variations with greater precision.
Traditional Methods for Analyzing Structural Heterogeneity
Traditional methods for analyzing structural heterogeneity typically rely on techniques like principal component analysis (PCA) or K-means clustering. These methods help in classifying particles into different conformational states by reducing the dimensionality of the data. However, they often struggle to distinguish between continuous conformational changes, particularly when there is no clear-cut boundary between states. This limitation is especially evident when studying dynamic systems that undergo gradual transitions between functional states.
For example, traditional clustering methods may fail to adequately capture the full range of conformational flexibility seen in proteins such as ion channels or the transitions in multi-subunit complexes like ribosomes during translation. As a result, these methods often provide oversimplified representations of structural heterogeneity.
AI Enhancements in Structural Heterogeneity Analysis
AI and machine learning techniques, particularly deep learning models, are now being employed to significantly improve the analysis of structural heterogeneity in cryo-EM data. These advancements help capture subtle conformational changes that are often missed by traditional methods, enabling a more accurate understanding of dynamic molecular systems.
| AI Tools | Description |
|---|---|
| Variational Autoencoders (VAE) | VAEs are a powerful tool for analyzing structural heterogeneity, especially for systems undergoing continuous conformational transitions. By reducing the data dimensionality, VAEs can identify intermediate states within dynamic complexes. For example, in the translation process of ribosomes, VAEs can recognize and separate distinct conformational states that occur between the canonical "open" and "closed" forms. This allows researchers to better visualize the functional dynamics of macromolecules during their biological processes. |
| Deep Learning Classification with 3D Convolutional Networks | Another significant advancement is the application of deep learning-based classification methods using 3D CNNs, such as CryoDRGN. These networks are particularly useful for separating different functional states of membrane proteins, such as ion channels transitioning between open and closed states. By training deep learning models on large datasets of particle images, AI can classify different conformations more accurately than traditional methods, providing more detailed insights into the functional dynamics of proteins. |
These AI-driven techniques offer significant advantages over traditional methods, enabling a more detailed and comprehensive understanding of structural heterogeneity. The ability to capture intermediate conformational states and differentiate between continuous changes provides a clearer picture of how macromolecules function in vivo.
Post-Processing and Map Sharpening
Post-processing and map sharpening are crucial steps in cryo-EM data analysis to improve the quality of the reconstructed 3D maps. These processes help to enhance the visibility of high-resolution structural features while minimizing noise. Traditional techniques have their limitations, especially when dealing with low-resolution data or noise in cryo-EM maps. AI-driven approaches, however, have significantly improved the effectiveness of post-processing and map sharpening, providing sharper, clearer density maps for further analysis.
Traditional Post-Processing Techniques
Traditional post-processing techniques often rely on Fourier filtering methods, such as CTFFIND, to correct for aberrations and improve map quality. These techniques are effective at removing certain types of noise and correcting the Contrast Transfer Function (CTF), but they have limitations. For example, Fourier filtering can inadvertently amplify noise, especially in low-resolution regions of the map (typically above 4 Å resolution). Additionally, these methods are less effective at enhancing high-resolution features, such as beta-sheet structures, where subtle details are crucial for accurate modeling and interpretation.
Moreover, traditional map sharpening techniques often struggle with balancing the suppression of noise while preserving the fine structural details in high-resolution regions, leading to suboptimal results.
AI Algorithms for Post-Processing and Map Sharpening
AI-based algorithms have significantly enhanced post-processing and map sharpening by improving noise reduction and highlighting fine details in cryo-EM maps. These methods allow for more effective retention of high-resolution information while minimizing the impact of noise, especially in challenging low-resolution areas.
| AI Tools | Description |
|---|---|
| DeepSharpen | DeepSharpen is an AI-driven method that combines denoising and edge enhancement to significantly improve the quality of cryo-EM maps. By focusing on high-frequency details, DeepSharpen can enhance the clarity of structural features like beta-sheet regions, which are often hard to visualize in low-resolution maps. This tool can sharpen density maps with higher accuracy, ensuring that key structural elements are preserved for downstream analysis, such as model fitting and interpretation. |
| DeepRes | It is a CNN-based AI method designed to address limitations in traditional local resolution estimation for cryo-EM maps. It effectively detects local variations in map quality, which can result from post-processing steps like filtering, sharpening, and noise reduction. By offering more precise resolution estimation, DeepRes helps improve the overall clarity and accuracy of cryo-EM models. However, further development is needed to refine its application as a universally accepted method in the field. |
These AI-driven methods offer clear advantages over traditional approaches, providing more precise control over map sharpening and post-processing steps. The ability to enhance high-resolution features, while minimizing noise and preserving key structural details, results in cleaner, more interpretable cryo-EM maps.
Automated Model Building and Structure Fitting
Model building and structure fitting in cryo-EM involve the placement of atomic models into a 3D electron density map. AI algorithms, such as Emap2sec and EMBuild, are now being used to automate this process. These tools utilize deep learning architectures to identify secondary structures and build atomic models more efficiently, especially in cases of low-resolution maps.
These AI-based approaches not only improve accuracy but also reduce the computational burden associated with manual model fitting, making it easier to build reliable atomic structures, even from intermediate-resolution maps.
Advantages and Challenges of AI in Cryo-EM
| Advantages | Challenges |
|---|---|
|
|
Future Directions and Innovations in AI for Cryo-EM
The integration of AI with cryo-EM is in its early stages, but the potential for groundbreaking advancements is vast. Some of the most promising trends include:
- AI-Driven Cryo-EM and Cryo-ET Integration: AI is set to enhance cryo-electron tomography (cryo-ET), enabling faster and more accurate 3D reconstructions of large and complex structures. The synergy between AI and cryo-EM will expand the scope of structural biology, allowing for the detailed study of molecular machinery that was previously difficult to analyze.
- Real-Time AI Processing: Future AI tools could allow real-time analysis of data as it is collected during cryo-EM experiments. This would enable immediate decision-making and adjustments during data acquisition, streamlining workflows and optimizing experimental outcomes.
- AI-Enhanced Dynamic Modeling: With advancements in AI technologies like AlphaFold, we are moving toward dynamic modeling of biomolecules in various states, including reaction intermediates and conformational changes. This capability could significantly impact drug discovery by providing more accurate models for molecular interactions.
- Multimodal AI Integration: The future may also see the integration of cryo-EM with other techniques such as X-ray crystallography and mass spectrometry. Combining these datasets will allow for more comprehensive all-atom models, enhancing the understanding of molecular structures at an unprecedented level.
- Quantum Computing and AI: The potential of quantum computing could further accelerate AI-driven cryo-EM. Quantum Generative Adversarial Networks (QGANs) are an exciting frontier, optimizing dimensionality reduction and enabling the processing of complex high-dimensional data more efficiently.
Select Service
In conclusion, the integration of AI and Cryo-EM marks the entry of structural biology into the era of intelligent analysis. From particle selection to dynamic conformation analysis, AI not only improves efficiency and accuracy, but also expands the boundaries of research (such as functional mechanisms at the single-molecule level). In the future, with algorithm optimization and interdisciplinary collaboration, AI will further promote new drug development (such as target-drug complex design) and disease mechanism research.
At Creative Biostructure, we provide comprehensive Cryo-EM solutions tailored to your research needs. Contact us to learn how we can help advance your research with our advanced cryo-EM capabilities.
References
- Wang F, Gong H, Liu G, et al. DeepPicker: A deep learning approach for fully automated particle picking in cryo-EM. Journal of Structural Biology. 2016, 195(3): 325-336.
- Zehni M, Do M N, Zhao Z. Deepsharpen: Deep-learning based sharpening of 3D reconstruction map from cryo-electron microscopy. 2020 IEEE 17th International Symposium on Biomedical Imaging Workshops (ISBI Workshops). IEEE, 2020: 1-4.
- Nguyen N P, Ersoy I, Gotberg J, et al. DRPnet: automated particle picking in cryo-electron micrographs using deep regression. BMC Bioinformatics. 2021, 22: 1-28.
- Leonhardt S A, Purdy M D, Grover J R, et al. CryoEM structures of the human HIV-1 restriction factor SERINC3 and function as a lipid transporter. bioRxiv. 2022: 2022.07. 06.498924.
- Chung J M, Durie C L, Lee J. Artificial intelligence in cryo-electron microscopy. Life, 2022, 12(8): 1267.
- Dhakal A, Gyawali R, Wang L, et al. Artificial Intelligence in Cryo-EM Protein Particle Picking: The Hope, Hype, and Hurdles. 2024.
- Liu Y T, Fan H, Hu J J, et al. Overcoming the preferred-orientation problem in cryo-EM with self-supervised deep learning. Nature Methods. 2024: 1-11.

-1.jpg)