Exosomes Target Delivery Based on Physical Modification
The natural targeting properties of exosomes have been discovered and recognized, but there are still many challenges in effective clinical therapy. Utilizing the natural targeting properties of exosomes alone does not allow for precise delivery of loaded drugs to specific sites and remains a great challenge in efficient gene/chemotherapy combination therapy. To overcome these difficulties, several approaches have been employed to engineer and modify exosomes to optimize the targeting, selectivity, and efficacy. Of these, physical modification is one of the commonly used exosome targeting methods.
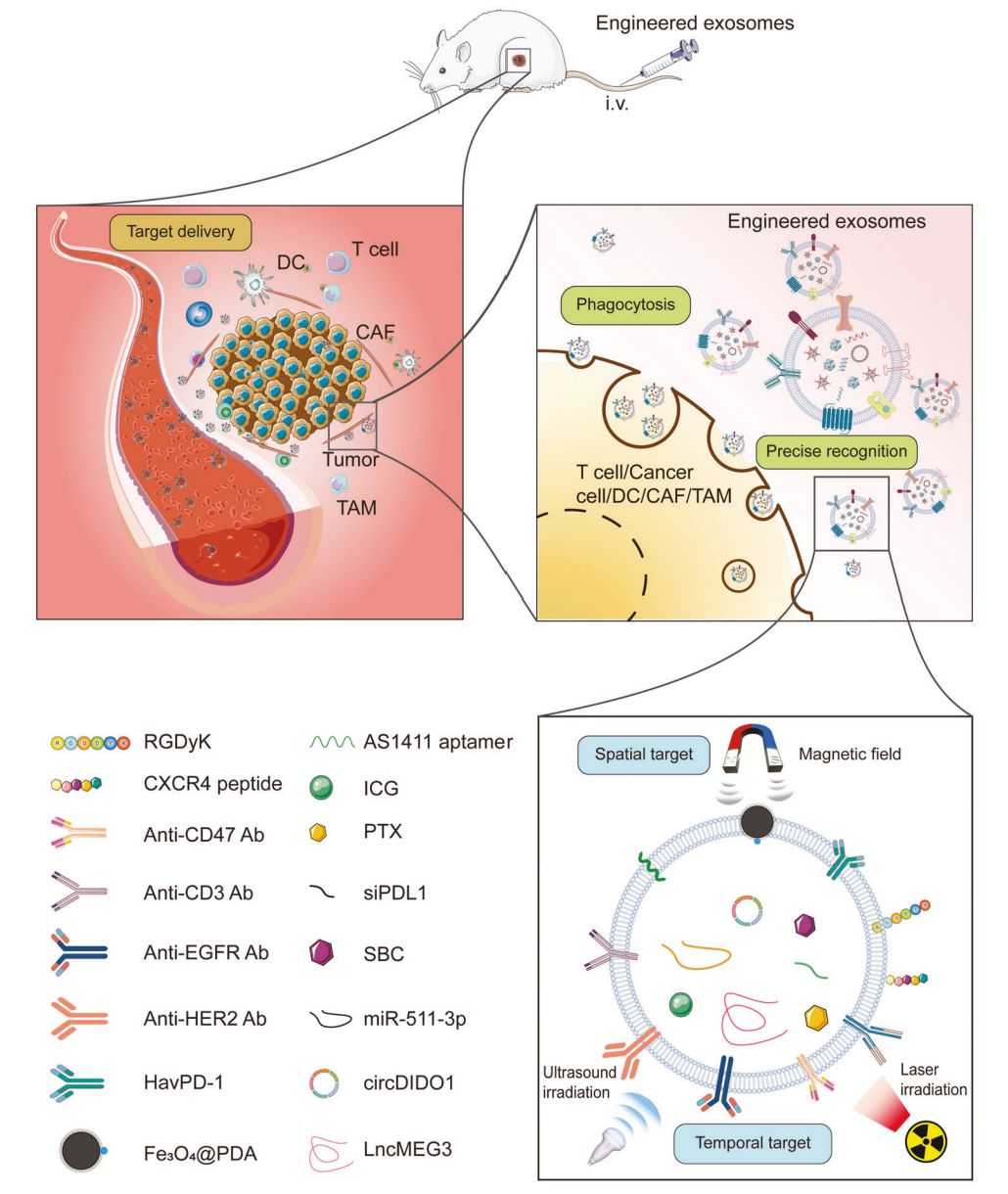 Figure 1. Engineered exosomes exhibiting antitumor effects on preclinical models. (Zhang M, et al., 2023)
Figure 1. Engineered exosomes exhibiting antitumor effects on preclinical models. (Zhang M, et al., 2023)
What is Exosome Targeting by Physical Modification?
Physical modification refers to the stimulation of exosomes by physical stimuli (including electric, ultrasound, magnetic fields, etc.) to affect certain signaling pathways, which in turn regulate the expression of proteins on the surface of exosomes. For example, mechanical methods that use superparamagnetic nanoparticles to capture exosomes and apply a magnetic field at the tumor site to improve tumor targeting. In addition to magnetic targeting, external laser irradiation can likewise achieve high tumor-targeting therapeutic efficacy, especially in photothermal therapy (PTT). Similarly, external near-infrared radiation (NIR) can be controlled to trigger the release of chemotherapeutic agents.
What are the Common Methods of Physical Modification?
- Electrostatic Action
Electrostatic action utilizes the principle of oppositely charged attraction. A charge is applied to the surface of the exosome membrane, thereby increasing the targeting efficiency of the exosome to the heterogeneously charged biofilm. For example, fusion of cationic lipid (CL) to the surface of exosomes facilitates the intercellular uptake and intracellular release of exosomes encapsulating dextran macromolecules (70kD).
- Hydrophobic Interaction / Membrane Fusion
Due to the phospholipid bilayer structure on the surface, exosomes can be attached to liposomes through hydrophobic interaction. Liposomes are attached to the targeting peptide or polyethylene glycol and then fused to the liposome membrane by freeze-thawing without altering the glycoproteins on the surface of the exosome, thus obtaining exosomes with targeting effects.
- Magnetic Guidance
Tissue-targeted delivery can be achieved by modifying exosomes with magnetic nanoparticles. For example, bifunctional superparamagnetic nanoparticle exosomes. As a targeted drug delivery vehicle for cancer therapy, these exosomes exhibit superparamagnetism at room temperature and are more responsive to external magnetic fields than single superparamagnetic nanoparticles, which enhance the targeting of tumors and inhibit tumor growth under the influence of external magnetic fields.
- Acoustic Guidance
Using focused ultrasound it is possible to guide the delivery of acoustic-sensitive exosomes at specific sites. By loading sin porphyrin sodium (DVDMS) on homotypic tumor cell-derived exosomes, functionalized intelligent acoustic-sensitive exosomes (EXO-DVDMS) are formed. The homogeneous tumor targeting of EXO-DVDMS can then be noninvasively enhanced using an in vitro ultrasound device, providing a new idea for guided targeting of exosomes.
- Metal Particle Modification
Gold nanoparticles (AuNPs) are widely used in the medical industry for their multifunctional properties in therapeutics, imaging, and surface modification. The synthesized AuNPs are mechanically or extruded onto the membrane surface of exosomes, and in vivo experiments demonstrated that exosomes modified with AuNPs have unique brain-targeting properties.
Application Cases of Exosome Targeting by Physical Modification
In recent years, researchers have designed AExs, a platform for exosome modification with an active targeting approach consisting of a membrane anchor (BODIPY)-spacer (PEG)-targeting ligand (cyclic RGD peptide) (ASL). The ASL modification enhances the stability of the active targeting and introduces a bioimaging modality. Research has shown that AExs target B16F10 melanoma tumor sites through specific interactions between cyclic RGDs and integrins. Adriamycin-coated AExs (dAExs) significantly inhibited melanoma growth. The ASL modification transforms exosomes into a novel therapeutic vehicle that uniquely combines in vivo tracking and robust targeting with drug delivery.
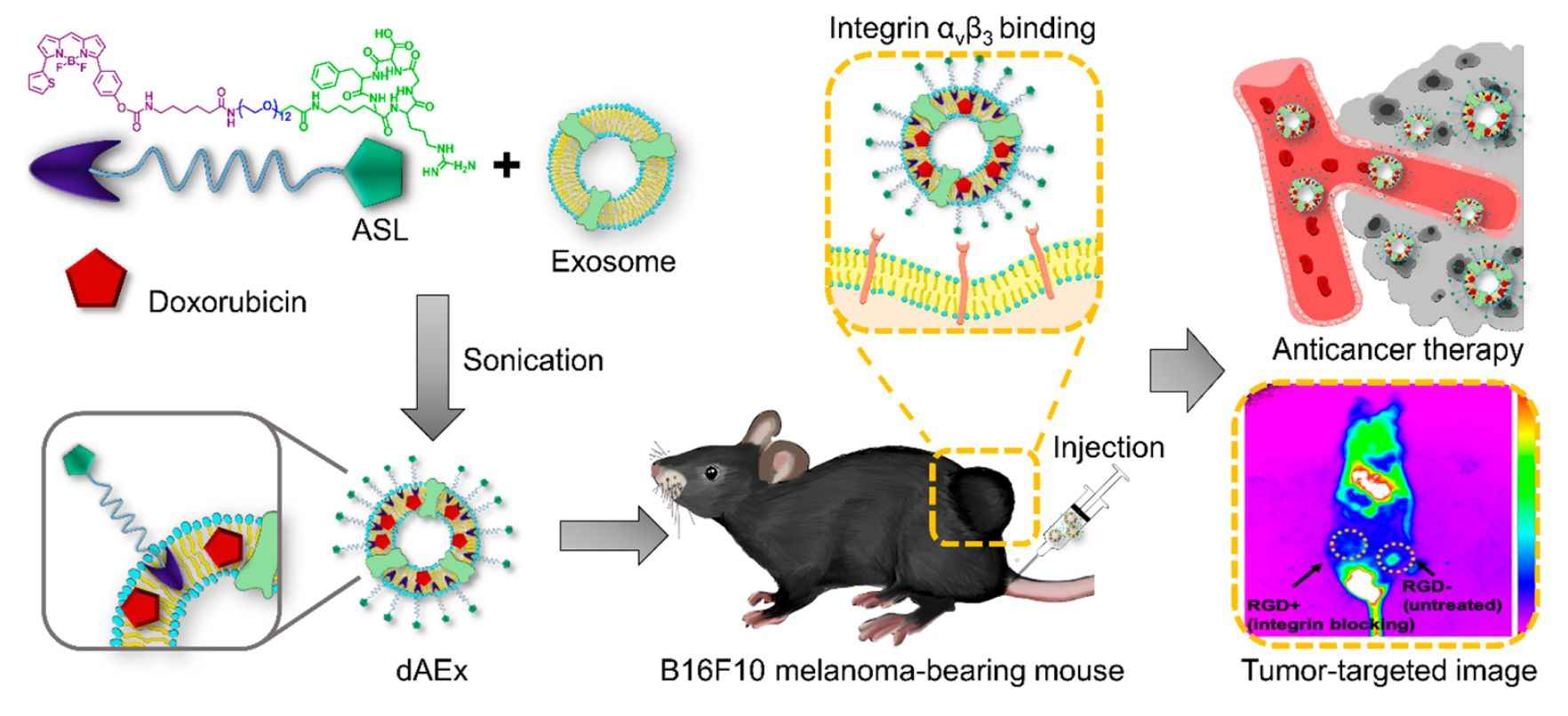 Figure 2. Schematic of ASL-functionalized exosome as a theragnostic agent for cancer therapy. (Kang C, et al., 2020)
Figure 2. Schematic of ASL-functionalized exosome as a theragnostic agent for cancer therapy. (Kang C, et al., 2020)
Neutrophils are the most abundant innate immune cells in the human circulation. In recent years, researchers have reported that exosomes from neutrophils (N-Ex) induce apoptosis in tumor cells by delivering cytotoxic proteins and activating the caspase signaling pathway. In addition, modification of N-Ex by superparamagnetic iron oxide nanoparticles (SPION) achieved higher tumor-targeting therapeutic efficacy. Under an external magnetic field, the DOX-loaded SPION-modified exosomes selectively accumulate at the tumor site, effectively inhibiting tumor growth.
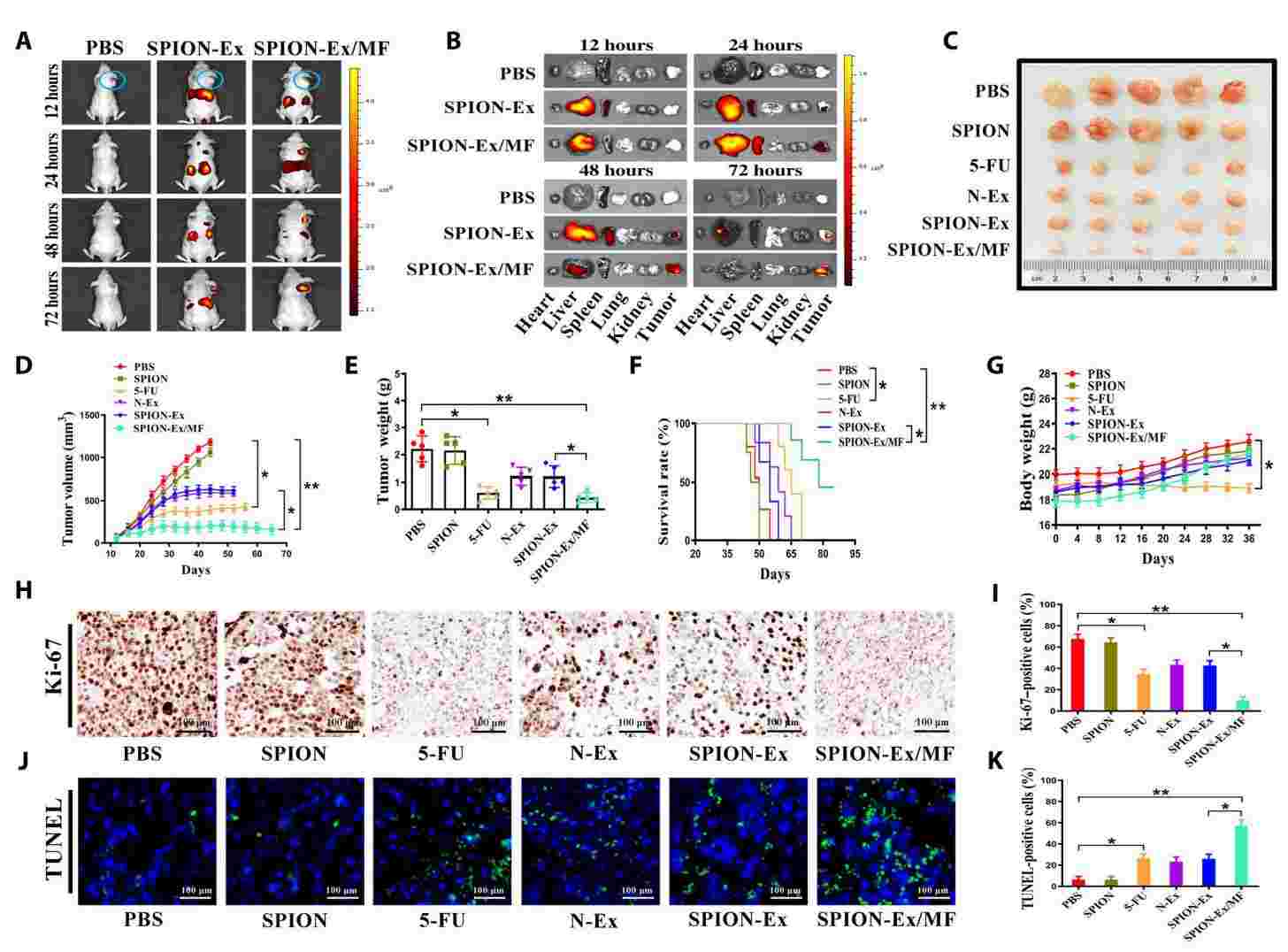 Figure 3. Tumor-targeting ability and the therapeutic effect of SPION-Ex in vivo. (Zhang J, et al., 2022)
Figure 3. Tumor-targeting ability and the therapeutic effect of SPION-Ex in vivo. (Zhang J, et al., 2022)
Our Products
We have decades of research experience in the field of exosomes and offer exosome targeting services based on physical modifications to enhance exosome specificity for therapeutic and diagnostic purposes. In addition, we provide a comprehensive range of high-purity exosome products directly for exosome modification to explore the potential capabilities of exosomes in precision cancer therapy.
| Cat No. | Product Name | Source |
| Exo-CH04 | HQExo™ Exosome-H196 | Exosome derived from Human small cell lung cancer cell line (H196 cell line) |
| Exo-CH05 | HQExo™ Exosome-H841 | Exosome derived from human small cell lung cancer cell line (H841 cell line) |
| Exo-CH06 | HQExo™ Exosome-MCF-7 | Exosome derived from human breast cancer, noninvasive cell line (MCF-7 cell line) |
| Exo-CH07 | HQExo™ Exosome-MDA-MB-231 | Exosome derived from human breast cancer, aggressive/invasive/metastatic cell line (MDA-MB-231 cell line) |
| Exo-CH09 | HQExo™ Exosome-DAUD1 | Exosome derived from human burkitt lymphoma cell line (DAUD1 cell line) |
| Exo-CH10 | HQExo™ Exosome-HCT116 | Exosome derived from human colorectal carcinoma cell line (HCT116 cell line) |
| Exo-CH16 | HQExo™ Exosome-COLO1 | Exosome derived from human colon carcinoma (COLO1 cell line) |
| Exo-CH17 | HQExo™ Exosome-HT29 | Exosome derived from human adenocarcinoma (HT29 cell line) |
| Exo-CH23 | HQExo™ Exosome-BxPC-3 | Exosome derived from human pancreas carcinoma cell line (BxPC-3 cell line) |
| Explore All Exosomes Isolated from Cancer Cell Lines | ||
Creative Biostructure's exosome targeting services are designed to improve the stability and therapeutic efficiency of exosomes as drug carriers. We are committed to driving innovation and creating impactful solutions through cutting-edge technology. If you are interested in our exosome products and services, please feel free to contact us for more details.
References
- Zhang M, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023. 8(1): 124.
- Kang C, et al. Anchor, Spacer, and Ligand-Modified Engineered Exosomes for Trackable Targeted Therapy. Bioconjug Chem. 2020. 31(11): 2541-2552.
- Zhang J, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022. 8(2): eabj8207.
- He J, et al. Exosomal targeting and its potential clinical application. Drug Deliv Transl Res. 2022. 12(10): 2385-2402.