Structural Research of Liponucleotide Synthetases
Liponucleotide synthetases are fundamental enzymes responsible for the biosynthesis of crucial phospholipids involved in biological membranes. Among these, phosphatidylinositol, phosphatidylglycerol, and phosphatidylserine play pivotal roles in cellular functions. Understanding the structural aspects of these enzymes and their catalytic mechanisms is essential for elucidating membrane dynamics and signal transduction in living organisms.
Recent breakthroughs in structural biology have shed light on the atomic-level details of liponucleotide synthetases. A recent study focused on the Cds from Thermotoga maritima (TmCdsA) has provided invaluable insights. TmCdsA forms a homodimer, with each monomer comprising nine transmembrane helices arranged in a novel fold with three domains.
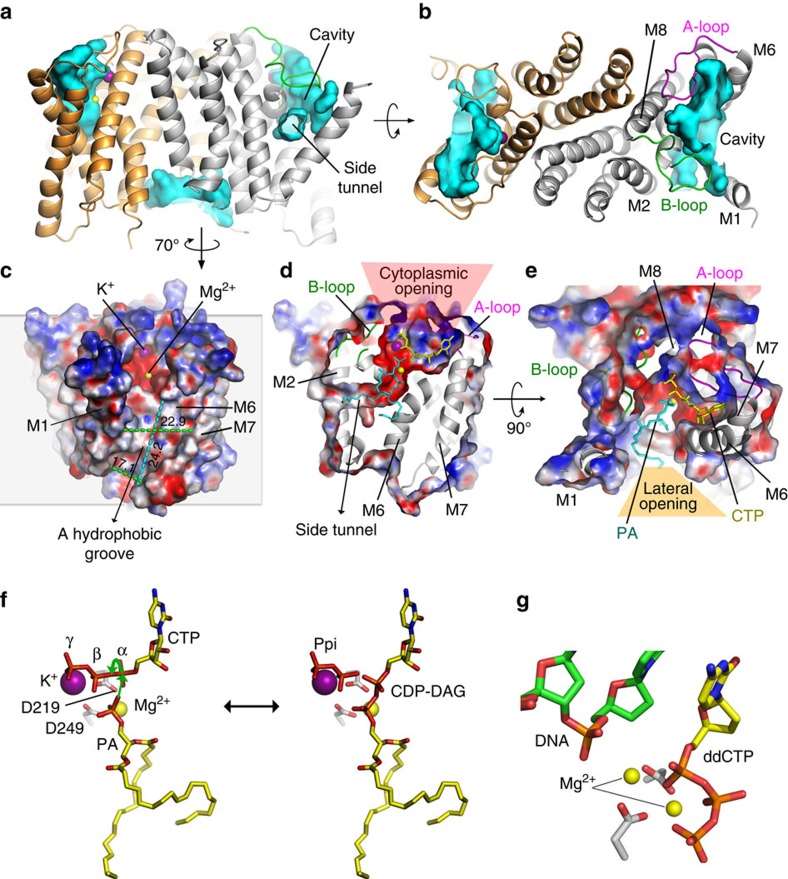 Figure 1. The substrate binding cavity of TmCdsA and its catalytic mechanism. (Liu X, et al., 2014)
Figure 1. The substrate binding cavity of TmCdsA and its catalytic mechanism. (Liu X, et al., 2014)
Membrane-Cytoplasm Interface: A Catalytic Hub
A fascinating characteristic of TmCdsA is the presence of an unusual funnel-shaped cavity penetrating halfway into the membrane. This unique feature enables the enzyme to simultaneously accept the hydrophilic substrate (cytidine 5′-triphosphate (CTP)/deoxy-CTP) from the cytoplasm and the hydrophobic substrate (phosphatidic acid) from the membrane. This spatial arrangement plays a critical role in facilitating phosphoinositide recycling during signal transduction, a fundamental process for cellular communication.
Cofactor and Catalytic Mechanism
Within the bottom of the cavity, a Mg2+-K+ hetero-di-metal center coordinated by an Asp-Asp dyad serves as the cofactor of TmCdsA. This cofactor is essential for the catalytic mechanism of Cds-mediated synthesis of CDP-DAG. The discovery of a two-metal-ion catalytic mechanism at the membrane-cytoplasm interface unveils a previously unexplored aspect of membrane-associated enzymatic reactions. TmCdsA forms a homodimer with each monomer consisting of nine transmembrane helices arranged into a novel fold with three domains. This unique arrangement provides significant clues about the enzyme's function and interactions with substrates.
Structural Insights into CDP-DAG Biosynthesis
A crucial step in phosphoinositide recycling during signal transduction involves the formation of CDP-DAG, an intermediate in the biosynthesis of phosphatidylinositol. These research findings suggest that TmCdsA employs a two-metal-ion catalytic mechanism to synthesize CDP-DAG at the interface between the membrane and the cytoplasm.
The most striking feature of TmCdsA is its funnel-shaped cavity that penetrates halfway into the membrane. This extraordinary characteristic enables the enzyme to accept both hydrophilic substrates (cytidine 5′-triphosphate (CTP)/deoxy-CTP) from the cytoplasm and hydrophobic substrates (phosphatidic acid) from the membrane concurrently. The Mg2+-K+ hetero-di-metal center, coordinated by an Asp-Asp dyad located at the bottom of the cavity, acts as the essential cofactor for TmCdsA, facilitating the catalytic reaction.
| Protein | Organism | Method | Resolution | PDB Entry ID |
| CDP-DAG synthetase, S200C/S258C active mutant (expressed in Escherichia coli) | Thermotoga maritima MSB8 | X-ray diffraction | 3.40 Å | 4Q2E |
Table 1. Structural research of liponucleotide synthetases.
Creative Biostructure, a pioneering leader in structural biology, offers cutting-edge services for structural analysis of biomolecules, including liponucleotide synthetases. Utilizing state-of-the-art technologies such as X-ray crystallography, cryo-electron microscopy, and NMR spectroscopy, our expert team of scientists is proficient in elucidating high-resolution structures of integral membrane proteins. Contact us to discover how our cutting-edge capabilities can empower your research and drive you closer to achieving your scientific goals.
Reference
- Liu X, et al. Structure and mechanism of an intramembrane liponucleotide synthetase central for phospholipid biosynthesis. Nature Communications. 2014, 5(1): 4244.