Structural Research of Neurotransmitter Sodium Symporter (NSS) Family
The neurotransmitter sodium symporter (NSS) family plays an important role in signaling. NSS takes up a variety of bioamines, penetrants, amino acids, and related nitrogenous substances through solute and Na+ interactions in a sodium - and chlorine-dependent manner. The NSS family is a member of the APC superfamily. It is widely found in bacteria, archaea and eukaryotes. In recent years, studies have shown that NSS is a target for antidepressants, psychostimulants, and other psychotropic drugs.
Research Progress of NSS in Prokaryotes
NSS in bacteria is Cl− dependent. The structural surface of bacterial NSS LeuT binds two Na ions at the Na1 and Na2 sites, and the substrate leucine binds to the binding site located in the center. Cl− interacts with the side chain hydroxyl groups of Tyr47, Ser290, and Thr254 in LeuT and the side chain amides of Gln250. The crystal structure shows that LeuT is a closed binding bag containing L-leucine (Leu) and two Na +. The two Na + recombine under the main chain and side chain interaction.
Research Progress of NSS Structure
The dynamics of ligand motion in LeuT were studied using the SMD model to simulate crystal structure. It is found that transmembrane segments TM1, TM3, TM6 and TM8 are closer to the structure. dDAT and hSERT are eukaryotic NSS. The N-terminal in their crystal structure begins as a conserved near-membrane motif that interacts with the intracellular rings IL-3, IL4, and IL5 and regulates transporter conformation, bottom flow out, and input. The structure of dDAT and hSERT shows a ten-residue helical "latch" at the C-terminal, which interacts with IL1 and regulates protein folding and transport.
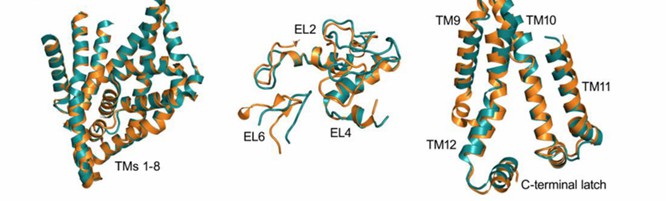 Figure 1. The structure of dDAT and hSERT. (Navratna V, et al., 2019)
Figure 1. The structure of dDAT and hSERT. (Navratna V, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| LEUTAA, a bacterial homolog of Na+/Cl--dependent neurotransmitter transporters | Aquifex aeolicus VF5 | X-ray diffraction | 1.65 Å | 2A65 |
| LeuT mutant F259V bound to sodium and L-tryptophan | Aquifex aeolicus | X-ray diffraction | 2.631 Å | 3QS4 |
| LeuT mutant I359Q bound to sodium and L-tryptophan | Aquifex aeolicus | X-ray diffraction | 2.6 Å | 3QS5 |
| LeuT mutant F259V, I359Q bound to sodium and L-tryptophan | Aquifex aeolicus | X-ray diffraction | 2.801 Å | 3QS6 |
| LeuT with V269 deletion | Aquifex aeolicus VF5 | X-ray diffraction | 2.615 Å | 6NLE |
| LeuT in an inward-facing occluded conformation | Aquifex aeolicus VF5 | X-ray diffraction | 2.6 Å | 6XWM |
| LeuT in lipidic cubic phase at pH 7 | Aquifex aeolicus | X-ray diffraction | 2.403 Å | 7DII |
| G26C mutant of LeuT | Aquifex aeolicus | X-ray diffraction | 3.528 Å | 7DJ1 |
| G26C/E290S mutant of LeuT | Aquifex aeolicus | X-ray diffraction | 2.4 Å | 7DJ2 |
| G26C/Q250A mutant of LeuT | Aquifex aeolicus | X-ray diffraction | 2.701 Å | 7DJC |
| LeuT bound to L-Alanine | Aquifex aeolicus VF5 | X-ray diffraction | 2.144 Å | 7LQJ |
| R375A mutant of LeuT | Aquifex aeolicus VF5 | X-ray diffraction | 2.1 Å | 7LQK |
| R375D mutant of LeuT | Aquifex aeolicus VF5 | X-ray diffraction | 2.6 Å | 7LQL |
| Dopamine N Acetyltransferase in complex with acetyl-COA from Drosophila Melanogaster | Drosophila melanogaster | X-ray diffraction | 1.46 Å | 3TE4 |
| Drosophila melanogaster Dopamine N-Acetyltransferase Bound to CoA | Drosophila melanogaster | X-ray diffraction | 1.45 Å | 5GI5 |
Table 1. Structural research of neurotransmitter sodium symporter (NSS) family.
To study the structure of the neurotransmitter sodium symporter (NSS), X-ray crystallography is commonly used. Structural analysis of NSS proteins is beneficial to develop drugs for neuropsychiatric disorders.
Creative Biostructure has long been committed to the study of structural biology and membrane proteins. We have extensive experience in determining membrane protein structures.
In addition to the structural determination of membrane proteins, we can accurately analyze biomolecules, including but not limited to nucleic acids, ribosomes, small proteins, protein complexes, protein-ligand complexes, and viruses. If you are interested in our services, please contact us and we will provide you with a professional and comprehensive solution.
References
- Shi L, et al.The mechanism of a neurotransmitter: sodium symporter--inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30(6):667-677.
- Navratna V, et al. Insights into the mechanism and pharmacology of neurotransmitter sodium symporters. Curr Opin Struct Biol. 2019; 54:161-170.
- Kantcheva AK, et al.Chloride binding site of neurotransmitter sodium symporters. Proc Natl Acad Sci U S A. 2013;110(21):8489-8494.
