Labeling Exosomes with Near Infrared (NIR) Fluorescent Probes
Recognizing the biological, diagnostic, and medical essentiality of exosomes, there is an urgent need to efficiently label these extracellular vesicles in a way that does not alter their intrinsic properties. The development of near-infrared (NIR) fluorescent probes has transformed biomolecular imaging over the past decades and represents one of the fastest-growing research areas. NIR fluorescent probes are commonly used to visualize and understand exosome activity. With their ability to deeply penetrate tissues, reduce damage to exosomes, and high signal-to-noise ratios, they are effective, next-generation tools for elucidating various biological events.
What are NIR Fluorescent Probes?
NIR fluorescent probes are molecules that emit near-infrared light and are used in biological imaging and detection. They are usually composed of organic molecules or inorganic nanomaterials that can emit fluorescence at wavelengths between 650-900nm. This wavelength range has good penetration in biological tissues, thus NIR fluorescent probes have wide applications in in vivo imaging. In addition to labeling exosomes, NIR fluorescent probes can be used in a variety of biomedical research fields.
- In oncology, it can detect tumor boundaries and tumor blood vessels.
- In neuroscience, it can observe brain activity and neuronal connections.
- In immunology, it can track immune cells and immune responses.
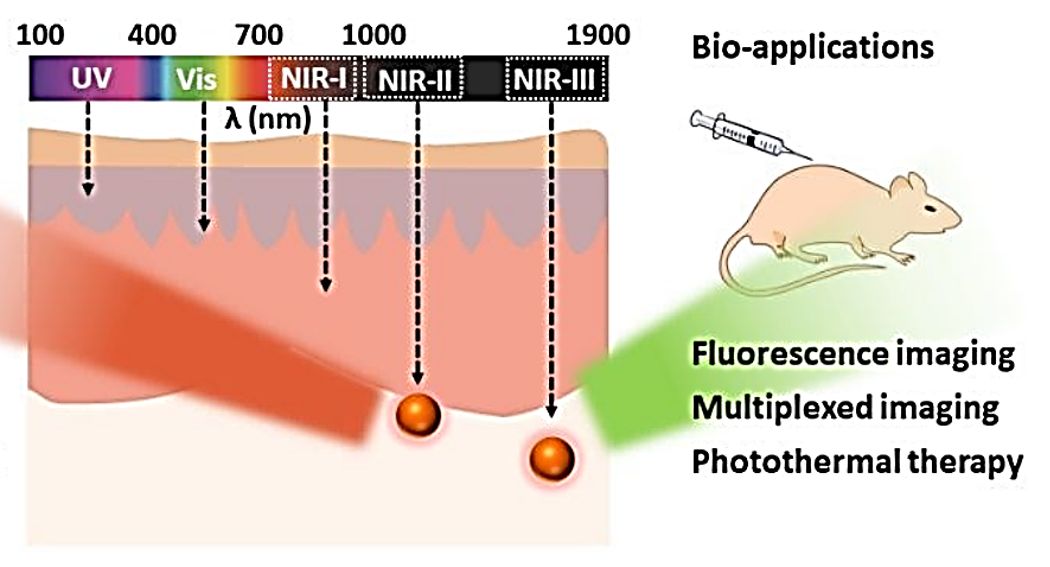 Figure 1. Absorption and emission wavelength regions of NIR fluorescent probes and their bio-applications. (Yang F, et al., 2020)
Figure 1. Absorption and emission wavelength regions of NIR fluorescent probes and their bio-applications. (Yang F, et al., 2020)
NIR fluorescent probes have the advantages of low background interference, high tissue penetration, and low tissue damage. Many fluorescent dyes, such as BODIPY, anthocyanin, rhodamine, and coumarin, have been used as fluorophores to design NIR fluorescent probes. Endogenous detection of NIR fluorescently labeled exosomes using amphiphilic probes eliminates the need for immunolabeling or chromatography of exosomes.
What are the Design Mechanisms of NIR Fluorescent Probes?
Common design mechanisms for NIR fluorescent probes include phototransfer of electrons (PET), excited state intramolecular proton transfer (ESIPT), fluorescence resonance energy transfer (FRET), transfer of energy through bonding (Tbet), internal charge transfer (ICT), twisted intramolecular charge transfer (TICT), and aggregation-induced emission (AIE). Of these, PET is the most classical. The PET mechanism can be explained based on the frontline orbital theory. When the probe is excited at an appropriate wavelength, the electrons occupying the highest molecular orbital (HOMO) are transferred to the lowest molecular orbital (LUMO). Since the HOMO energy level of the acceptor group is located between the two energy levels of the excited fluorophore, the electrons in the LUMO of the fluorophore cannot return to the original HOMO, resulting in the phenomenon of fluorescence burst. When the acceptor group forms a complex with the analyte, the HOMO energy level of the recognition group is lower than the HOMO energy level of the fluorophore, thus inhibiting the PET process, the electrons in the fluorophore return to the HOMO level, and fluorescence is restored.
New Options for Fluorescent Probes - Near Infrared-II (NIR-II) Fluorescent Dyes
In past work, researchers have mainly focused on fluorescence imaging in the visible region (400 nm-750 nm) and within the NIR-I window (700 nm-900 nm). However, the different scattering and absorption of excitation and emission light by components within the exosome have resulted in a relatively low optical penetration depth and imaging resolution within these two intervals. Recently, researchers have found that within the near-infrared second window region (NIR-II, wavelengths 1000 nm-1700 nm), biological tissues have extremely low scattering and absorption of excitation light and fluorescence signals. Compared with the visible region and NIR-I, fluorescence signals within the NIR-II window enable better exosome imaging. Meanwhile, the autofluorescence effect of exosomes in the NIR-II region is also lower, and the "noise" in imaging is significantly reduced. Fluorescence imaging in the NIR-II region has a better performance and a great development prospect in the multi-detection of biological tissues.
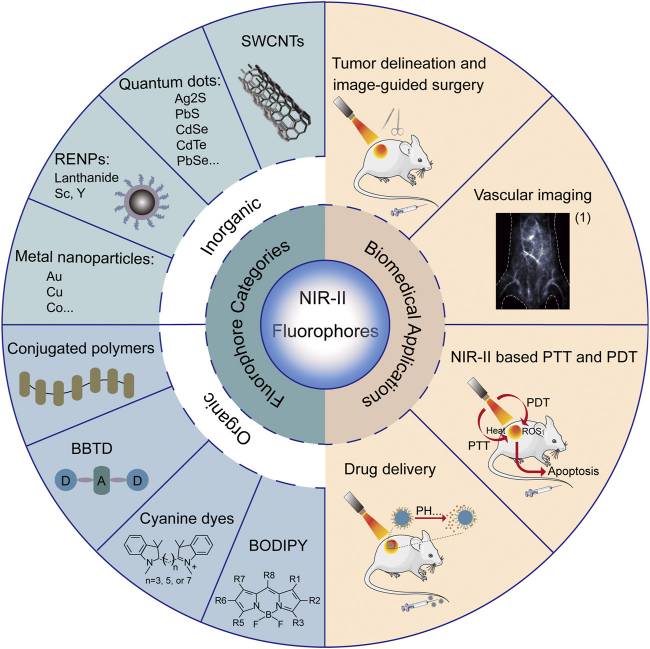 Figure 2. Summary of categories and biomedical applications of NIR-II fluorophores. (Chen Y, et al., 2021)
Figure 2. Summary of categories and biomedical applications of NIR-II fluorophores. (Chen Y, et al., 2021)
Types of NIR-II Fluorophores
Fluorophores are the core and foundation of fluorescence imaging. By designing and optimizing fluorophores for imaging in the NIR-II window, their multifunctional use in tumor diagnosis and treatment can be realized. Currently, representative NIR-II fluorophores can be categorized according to their chemical structure as follows
- Inorganic - Single-walled carbon nanotubes (SWCNTs), quantum dots (QDs), and rare-earth doped nanoparticles (RENPs).
- Organic - Small molecular dyes (SMDs), aggregation-induced emission fluorogens (AIEgens), and conjugated polymers (CPs).
NIR-II fluorescence imaging is still in its infancy. There is a need to further develop novel NIR-II fluorescent probes with tunable emission wavelengths, high quantum yields, and low biotoxicity. Promising multifunctional NIR-II fluorescent probes will enable multimodal imaging and therapeutic diagnosis in clinical practice.
Advances in Research on Labeling Exosomes Using NIR Fluorescent Probes
Exosomes play essential roles in physiological and tumorigenic processes, thus there is a need to visualize and track exosomes. In recent years, researchers have reported a novel amphiphilic fluorescent probe, HBT-Exo, based on the ESIPT mechanism for exosome labeling. Both theoretical calculations and experimental observations confirmed its ESIPT properties, which enabled the probe to exhibit large Stokes shifts as well as near-infrared (NIR) keto-type emission. BT-Exo showed excellent biocompatibility and remarkable efficiency for exosome labeling in gastric cancer cells.
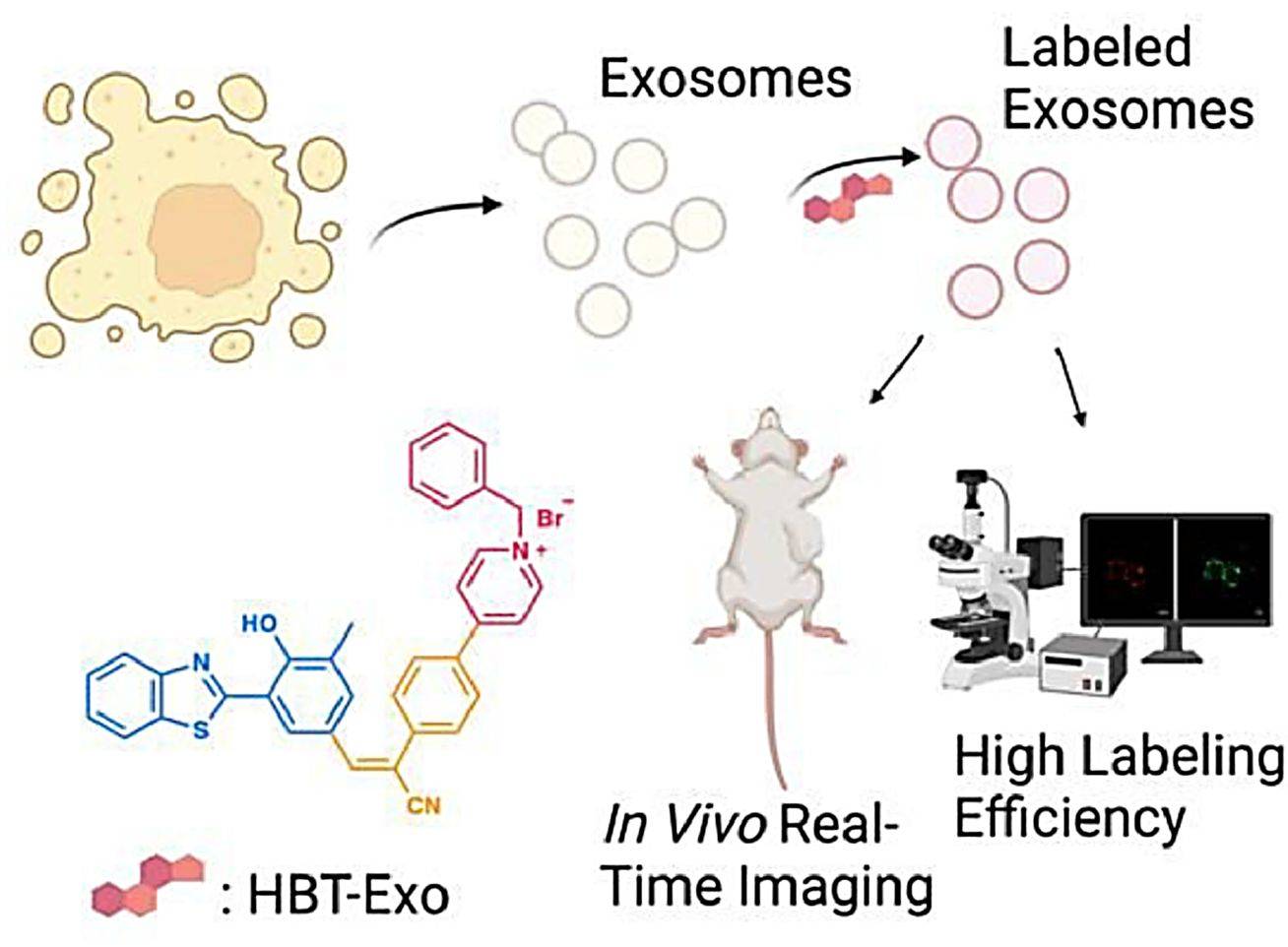 Figure 3. Schematic illustration of HBT-Exo as a fluorescence exosome-labeling tool. (Ding, J, et al., 2023)
Figure 3. Schematic illustration of HBT-Exo as a fluorescence exosome-labeling tool. (Ding, J, et al., 2023)
Mesenchymal stem cell-derived exosomes (MSC-Exos) are emerging as promising platforms for the treatment of a wide range of difficult diseases and organ damage. Monitoring their migration and therapeutic capacity in vivo is essential for the development of exosome-based therapeutic diagnostics. Researchers designed fluorescent semiconductor polymer dots (Pdot) in NIR-II for bright labeling and tracking of MSC-Exos. The glucose-coated Pdots (Pdots-Glu) are capable of labeling MSC-Exos without altering their biological properties. NIR-II fluorescent Pdot enables high labeling brightness and long-term in vivo tracking of MSC-Exos. The research suggests that the MSC-Exos therapeutic system has a promising application in liver regenerative medicine.
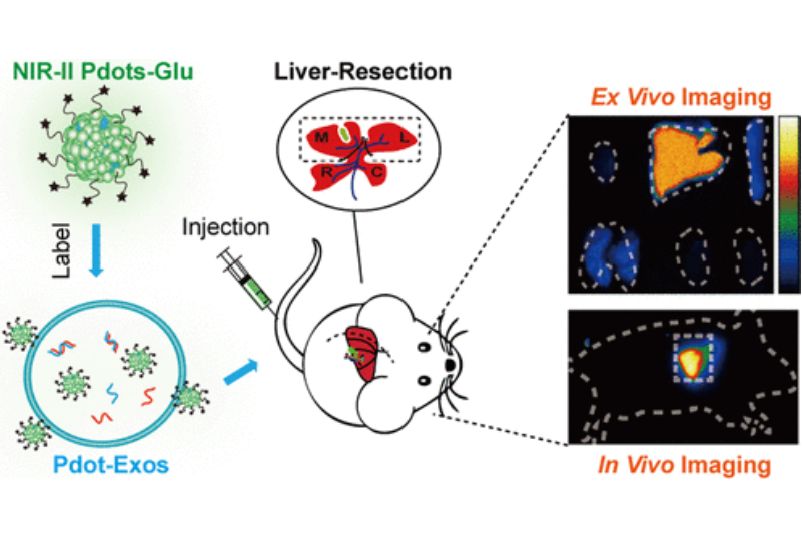 Figure 4. Schematic diagram of Pdots-Glu labeled MSC-Exos. (Ma N, et al., 2022)
Figure 4. Schematic diagram of Pdots-Glu labeled MSC-Exos. (Ma N, et al., 2022)
We offer a range of high-quality exosomes isolated from stem cell lines for clients.
| Cat No. | Product Name | Source |
| Exo-SC04 | HQExo™ Exosome-MSC | Exosome derived from Xeno-Free Human Mesenchymal Stem/Stromal Cells and Media |
| Exo-SC01 | HQExo™ Exosome-PCS-500-011 | Exosome derived from human pre-adipose derived mesenchymal stem cell (PCS-500-011) |
| Exo-SC03 | HQExo™ Exosome-hTERT | Exosome derived from hTERT-immortalized Mesenchymal Stem Cell |
| Exo-SC02-2 | HQExo™ Exosome-Pla-MSC | Exosome derived from human placental derived mesenchymal stem cell |
| Explore All Exosomes Isolated from Stem Cell Lines | ||
Why Choose NIR Fluorescent Probes to Label Exosomes?
- Low scattering effect - Scattering causes photons to deviate from their original path and reduces penetration depth. As the wavelength increases, the scattering coefficient of exosomes decreases, resulting in greater imaging depth and signal-to-noise ratio.
- Small tissue absorption - Many endogenous molecules within the exosome absorb visible light, causing attenuation of the fluorescence signal. The NIR-II imaging region has less absorption and less signal attenuation.
- Low autofluorescence - As the wavelength increases to the NIR-II region, the exosome autofluorescence decreases dramatically, resulting in a high signal-to-noise ratio.
In addition to exosome labeling services, we provide isolation, characterization, tracking, targeting, and functional analysis of exosomes to research their potential applications as diagnostics and therapeutics for cancer and chronic diseases. We also provide our clients with high-quality exosome kits and exosome products.
Creative Biostructure has extensive experience in exosome research. With years of successful projects and industry leadership, we can provide reliable and innovative solutions for our clients. Please feel free to contact us for a detailed quote.
References
- Yang F, et al. Recent advances of near infrared inorganic fluorescent probes for biomedical applications. J Mater Chem B. 2020. 8(35): 7856-7879.
- Chen Y, et al. Recent Advances in Second Near-Infrared Region (NIR-II) Fluorophores and Biomedical Applications. Front Chem. 2021. 9: 750404.
- Ding, J, et al. An ESIPT-based NIR-fluorescent probe for exosome labeling and in situ imaging. Chinese Chemical Letters. 2023. 34(11): 1001-8417.
- Ma N, et al. In Vivo Imaging of Exosomes Labeled with NIR-II Polymer Dots in Liver-Injured Mice. Biomacromolecules. 2022. 23(11): 4825-4833.