Antibodies, also known as immunoglobulins, are specialized proteins produced by the immune system in response to the presence of foreign substances called antigens. These antigens can be pathogens such as viruses, bacteria, or toxins. Antibodies play a critical role in the body's immune defense by recognizing and binding to these antigens, neutralizing them, or marking them for destruction. They are essential components of the adaptive immune system, which allows the body to target and remember specific pathogens, providing long-term immunity.
Here, Creative Biostructure provides a comprehensive guide to antibody structure, functions, and applications in research and diagnostics. We are experts in structural biology, and offer related services such as crystallization of antibody-antigen complexes and cryo-EM for antigen-antibody complexes.
Overview of Antibodies
Antibodies, also known as immunoglobulins, are specialized proteins produced by the immune system in response to the presence of foreign substances, known as antigens. These antigens could be pathogens such as viruses, bacteria, or toxins. Antibodies play a critical role in the body's immune defense by recognizing and binding to these antigens, neutralizing them, or marking them for destruction. They are essential components of the adaptive immune system, which allows the body to target and remember specific pathogens, providing long-term immunity.
Definition of Antibody
An antibody is a Y-shaped glycoprotein produced by B lymphocytes (a type of white blood cell) in response to exposure to an antigen. The primary function of the antibody is to recognize and bind to the specific antigen, thereby initiating an immune response to eliminate the pathogen. Each antibody is unique and specific for a particular antigen.
Importance in the Immune System
Antibodies are an integral part of the immune system's ability to fight infection. They function by recognizing antigens through specific molecular interactions that activate immune mechanisms such as phagocytosis, complement activation, and antibody-dependent cellular cytotoxicity. These mechanisms work together to neutralize and clear pathogens from the body. In addition, antibodies form the basis of vaccines and provide long-term immunity by preparing the body to recognize and fight specific pathogens.
Antibody Structure
Basic Structure of Antibodies
The basic structure of an antibody is typically described as a Y-shaped glycoprotein composed of four polypeptide chains. These include two identical heavy chains and two identical light chains. The chains are held together by disulfide bonds, which provide stability to the molecule.
- Y-shaped Glycoprotein Composed of Four Polypeptide Chains: The antibody molecule is structured to provide both flexibility and stability. The Y shape is essential for its function, with the arms of the Y containing the antigen binding sites and the base of the Y (the Fc region) interacting with immune cells and effector proteins.
- Heavy and Light Chains: The heavy chains are larger polypeptides, while the light chains are smaller. Each antibody contains two heavy chains and two light chains. These chains are critical in forming the antigen binding sites and the overall structure that enables antigen recognition.
- Variable (Fab) and Constant (Fc) Regions: Antibodies are divided into two major regions: the variable (Fab) region and the constant (Fc) region. The Fab region is responsible for antigen binding and contains the paratope (the specific site on the antibody that binds to the epitope of the antigen). The Fc region plays a role in the immune response by binding to receptors on immune cells to activate various immune processes.
Functional Regions
- Antigen-Binding Sites (Paratopes) in the Fab Region: The antigen-binding site, or paratope, is located in the variable region of the antibody and is where the antibody binds to the specific antigen (epitope). This region is highly variable, allowing antibodies to recognize a wide range of different antigens. The specificity of this binding is critical to the immune system's ability to accurately target pathogens.
- Role of the Fc Region in Immune Response: The Fc region is the constant part of the antibody that is responsible for engaging other components of the immune system. It binds to Fc receptors on immune cells such as macrophages, neutrophils, and natural killer cells. This interaction is key to initiating processes like phagocytosis, antibody-dependent cellular cytotoxicity (ADCC), and the activation of the complement system.
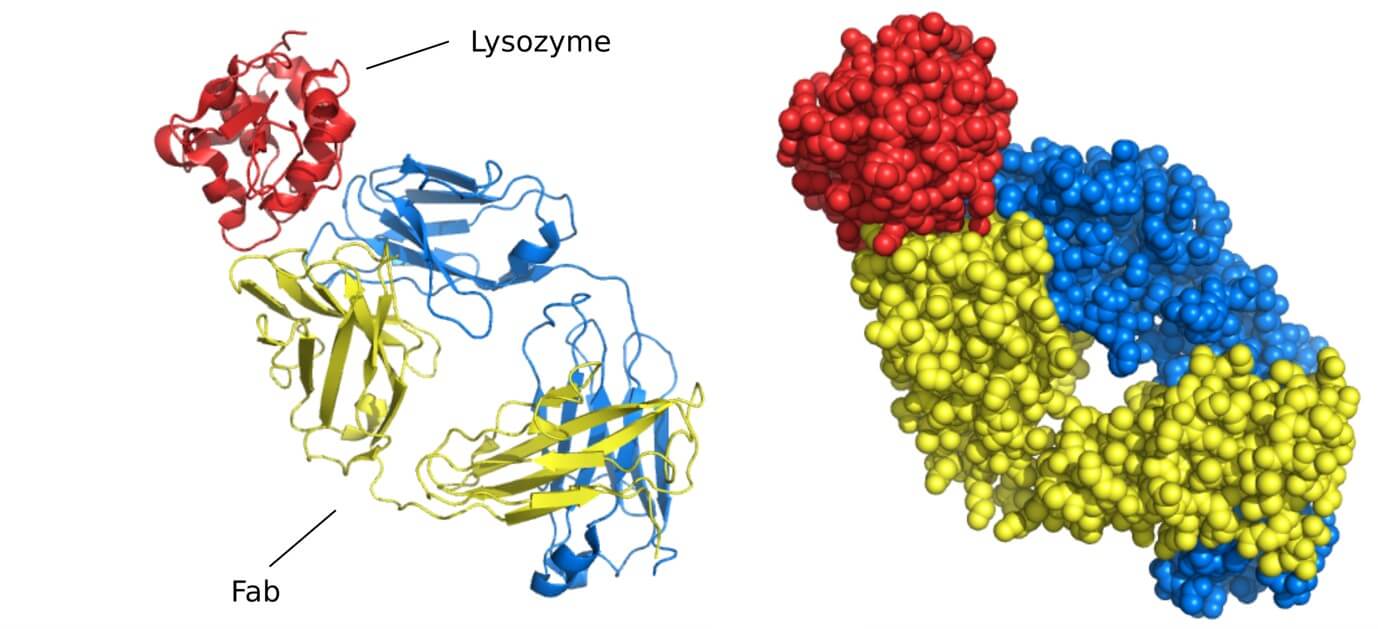
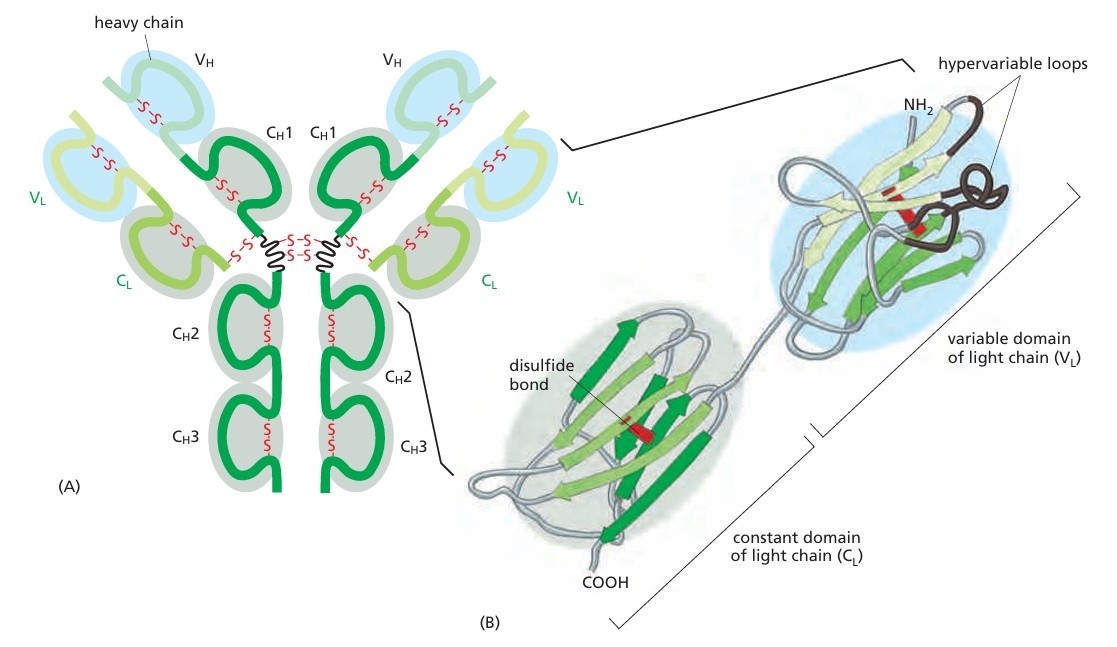 Figure 1. An antibody molecule. A typical antibody molecule is Y-shaped and has two identical binding sites for its antigen, one on each arm of the Y. The protein is composed of four polypeptide chains (two identical heavy chains and two identical and smaller light chains) held together by disulfide bonds. Each chain is made up of several different immunoglobulin domains, here shaded either blue or gray. The antigen-binding site is formed where a heavy-chain variable domain (VH) and a light-chain variable domain (VL) come close together. These are the domains that differ most in their sequence and structure in different antibodies. At the end of each of the two arms of the antibody molecule, these two domains form loops that bind to the antigen. (Molecular Biology of The Cell, 6th Edition).
Figure 1. An antibody molecule. A typical antibody molecule is Y-shaped and has two identical binding sites for its antigen, one on each arm of the Y. The protein is composed of four polypeptide chains (two identical heavy chains and two identical and smaller light chains) held together by disulfide bonds. Each chain is made up of several different immunoglobulin domains, here shaded either blue or gray. The antigen-binding site is formed where a heavy-chain variable domain (VH) and a light-chain variable domain (VL) come close together. These are the domains that differ most in their sequence and structure in different antibodies. At the end of each of the two arms of the antibody molecule, these two domains form loops that bind to the antigen. (Molecular Biology of The Cell, 6th Edition).
Related Reading
Antibody Function
Key Functions of Antibodies
Antibodies have several essential functions in the immune response. These functions ensure that the body can effectively combat and eliminate pathogens.
- Binding to Pathogens and Neutralizing Toxins: The primary function of antibodies is to bind to and neutralize pathogens (such as viruses and bacteria) or toxins. By binding to pathogens, antibodies prevent them from interacting with host cells, thereby inhibiting their ability to infect and cause disease. In the case of toxins, antibodies can neutralize their harmful effects.
- Activating the Immune System Through Phagocytosis: Once an antibody binds to a pathogen, it can activate phagocytosis, a process where immune cells such as macrophages engulf and destroy the pathogen. The Fc region of the antibody can bind to Fc receptors on phagocytic cells, triggering this response.
- Providing Long-Term Protection Against Pathogens: Antibodies provide long-lasting immunity by "remembering" specific pathogens. When the immune system encounters a pathogen for the first time, it produces antibodies. If the same pathogen invades again, memory cells rapidly produce antibodies, providing quicker and more effective protection.
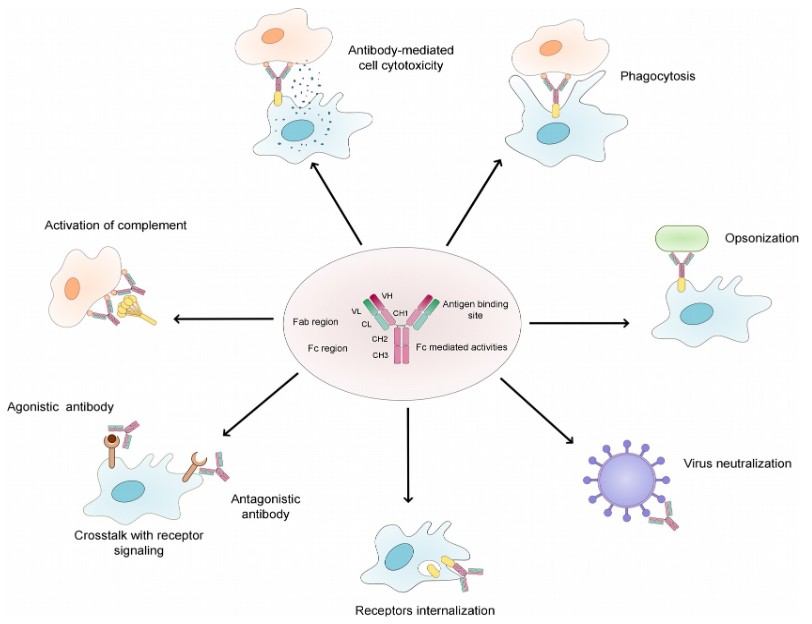 Figure 2. Overview of the natural function of antibodies. (Fesseha et al., 2020)
Figure 2. Overview of the natural function of antibodies. (Fesseha et al., 2020)
Mechanism of Action
- Binding to Antigens and Triggering Immune Responses: Antibodies bind to antigens with high specificity, and this interaction triggers various immune responses. For example, binding of antibodies to viral particles can prevent them from entering host cells, while binding to bacterial cells can initiate opsonization, marking the pathogen for destruction.
- Interaction with Effector Cells via the Fc Region: Once bound to the antigen, the Fc region of the antibody interacts with Fc receptors on effector cells such as macrophages, neutrophils, and natural killer cells. This interaction enhances the immune response, resulting in the elimination of the pathogen.
Main Types of Antibodies
Antibodies are classified into different isotypes based on their structure and function. These isotypes are differentiated by the constant region of the heavy chain and each has unique properties that allow it to carry out specific immune functions.
IgG–Most Abundant Antibody in Serum
IgG is the most abundant antibody in serum and plays a critical role in neutralizing pathogens. It is highly versatile and can cross the placenta to provide passive immunity to the fetus. IgG can also activate the complement system and mediate phagocytosis through Fc receptor binding.
IgM–First Response Antibody
IgM is the first antibody produced in response to an infection. It has high avidity for antigens, meaning it can bind tightly to pathogens. Although it is less versatile than IgG, IgM is highly effective in activating the complement system and agglutinating pathogens.
IgA–Found in Mucosal Areas
IgA is primarily found on mucosal surfaces such as the respiratory and gastrointestinal tracts, as well as in secretions such as saliva and breast milk. It plays a key role in preventing the colonization of pathogens on mucosal surfaces.
IgE–Involved in Allergic Reactions
IgE is primarily involved in allergic reactions and defense against parasitic infections. When IgE binds to allergens, it triggers the release of histamine from mast cells and basophils, leading to allergic symptoms. It also plays a role in defense against parasitic worms.
IgD–Unclear Function
IgD is found in low concentrations in the blood and is primarily expressed on the surface of B cells. Its exact function is still not fully understood, but it is thought to play a role in B cell activation and immune system regulation.
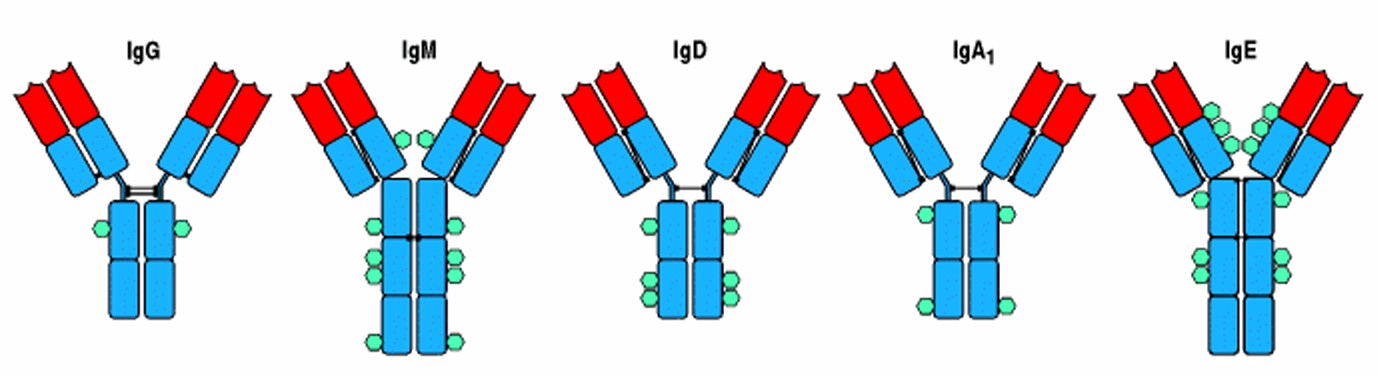 Figure 3. The structural organization of the main human immunoglobulin isotype monomers. Both IgM and IgE lack a hinge region but each contains an extra heavy-chain domain. Note the differences in the numbers and locations of the disulfide bonds (black lines) linking the chains. The isotypes also differ in the distribution of N-linked carbohydrate groups, shown as turquoise hexagons (Immunobiology, 5th Edition)
Figure 3. The structural organization of the main human immunoglobulin isotype monomers. Both IgM and IgE lack a hinge region but each contains an extra heavy-chain domain. Note the differences in the numbers and locations of the disulfide bonds (black lines) linking the chains. The isotypes also differ in the distribution of N-linked carbohydrate groups, shown as turquoise hexagons (Immunobiology, 5th Edition)
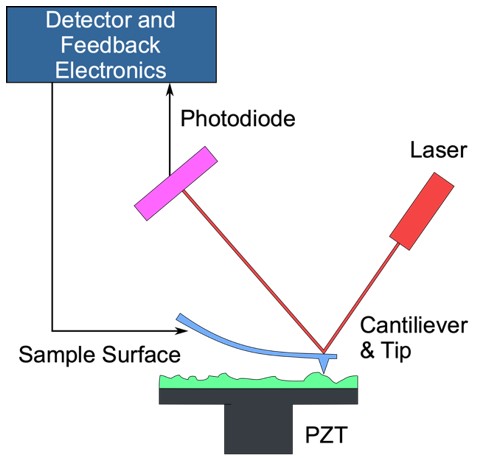
Analyzing Antibody Structure
Techniques for Studying Antibody Structure
| Techniques | Description |
|---|---|
| X-ray Crystallography | X-ray crystallography is a powerful technique used to determine the three-dimensional structure of antibodies at atomic resolution. By analyzing the diffraction patterns produced when X-rays pass through a crystal of the antibody, researchers can deduce its precise molecular structure. |
| Cryo-Electron Microscopy (Cryo-EM) | Cryo-EM is an emerging technique that allows the visualization of antibodies in a frozen, hydrated state. It provides high-resolution images and has become essential for the study of large and complex antibody structures. |
| Nuclear Magnetic Resonance (NMR) | NMR spectroscopy is used to study the dynamics and interactions of antibodies in solution. It provides valuable insight into the structure, conformation and binding mechanisms of antibodies. |
| Atomic Force Microscopy (AFM) | AFM is a nanoscopic imaging technique that enables the direct visualization of antibody molecules by scanning their surface topography. This method provides high-resolution three-dimensional images under near-physiological conditions without requiring extensive sample preparation. AFM is particularly useful for studying antibody interactions with ligands and cellular surfaces. |
| Transmission Electron Microscopy (TEM) | TEM is a powerful imaging technique for high-resolution visualization of antibodies and other macromolecules. By passing an electron beam through a thin sample, TEM produces detailed images of antibody morphology and aggregation states. It is widely used for structural characterization, but often requires staining techniques to enhance contrast. |
| Small-Angle Scattering (SAS) | Small-angle X-ray Scattering (SAXS) and small-angle neutron scattering (SANS) are techniques used to study the structure of antibodies in solution. These methods provide information about the overall shape, flexibility, and conformational changes of antibodies without the need for crystallization. SAXS and SANS are particularly useful for analyzing antibody-antigen interactions and dynamic structural changes. |
Select Service
Case Study
Case 1: Structure of Full-Length Human Anti-PD1 Therapeutic IgG4 Antibody Pembrolizumab
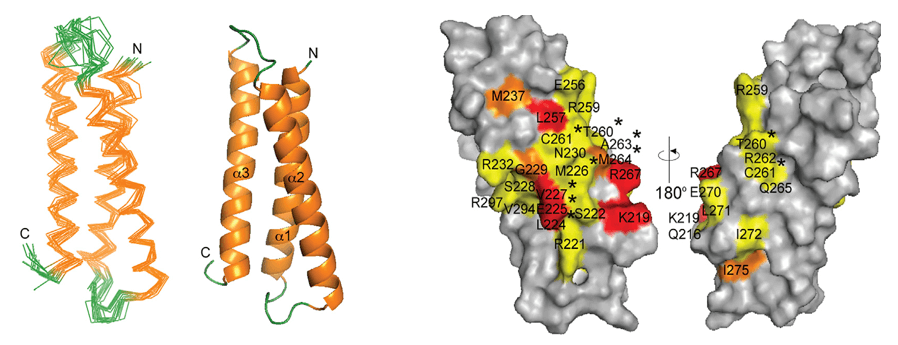
The crystallographic structure of the full-length human IgG4 S228P antibody pembrolizumab has been determined at a resolution of 2.3 Å. Pembrolizumab has a compact structure with a short hinge region that influences its molecular conformation. The Fc domain is glycosylated, with a CH2 domain rotated 20° compared to previously reported structures, likely due to the shorter hinge. The S228P mutation appears to prevent the exchange of the IgG4 arm. The structural analysis suggests potential diversity among IgG subclasses and highlights the limitations of using isolated antibody fragments. X-ray crystallography and NMR spectroscopy confirm the tight packing of Fab and Fc domains, which differs from the more flexible IgG1 monoclonal antibodies. The results are in agreement with small-angle X-ray scattering studies and reinforce the structural insights of the IgG subclasses.
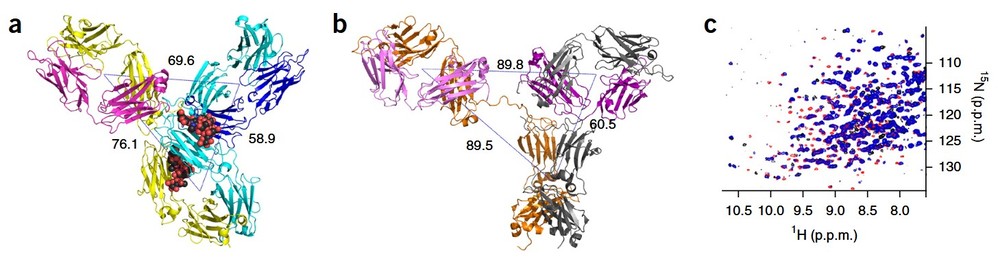 Figure 4. Overall pembrolizumab structure. (a) Pembrolizumab ribbon diagram showing the two heavy chains in yellow and cyan, and the two light chains in magenta (Fab1) and blue (Fab2). The sugars are shown as spheres colored by atom type (black, carbon; red, oxygen; blue, nitrogen). The distances between the center of mass of the three domains (Fab1, Fab2 and Fc), as calculated in PyMOL, are also shown. (b) Ribbon diagram of the structure of an IgG1 antibody (PDB 1HZH10); the distances between the center of mass of the three regions (Fab1, Fab2 and Fc) are shown. (c) 15N TROSY16 data for intact pembrolizumab (blue) are compared with data obtained from Fab (red) and Fc (black) fragments, showing differences in peak positions and intensities for residues in both the Fab and Fc domains. Neither the full-length nor the individual fragments have been assigned. (Scapin et al., 2015)
Figure 4. Overall pembrolizumab structure. (a) Pembrolizumab ribbon diagram showing the two heavy chains in yellow and cyan, and the two light chains in magenta (Fab1) and blue (Fab2). The sugars are shown as spheres colored by atom type (black, carbon; red, oxygen; blue, nitrogen). The distances between the center of mass of the three domains (Fab1, Fab2 and Fc), as calculated in PyMOL, are also shown. (b) Ribbon diagram of the structure of an IgG1 antibody (PDB 1HZH10); the distances between the center of mass of the three regions (Fab1, Fab2 and Fc) are shown. (c) 15N TROSY16 data for intact pembrolizumab (blue) are compared with data obtained from Fab (red) and Fc (black) fragments, showing differences in peak positions and intensities for residues in both the Fab and Fc domains. Neither the full-length nor the individual fragments have been assigned. (Scapin et al., 2015)
Case 2: Characterizing Monoclonal Antibody Formulations in Arginine Glutamate Solutions Using ¹H NMR Spectroscopy
This study investigates the use of solution NMR spectroscopy to assess monoclonal antibody (mAb) self-association in highly concentrated formulations, focusing on the effects of excipients like arginine glutamate. By analyzing 1D ¹H NMR spectra and accounting for buffer viscosity, the researchers identified conditions that minimize protein-protein interactions. While molecular translational diffusion rates were less effective, transverse relaxation rates proved useful for optimizing formulations. NMR also monitored solution viscosity and mAb aggregation, providing data consistent with size exclusion chromatography (SEC).
Accelerated stability studies of mAb2 examined its degradation at high concentrations (300 mg/ml) under different formulations at 40°C, using NMR and SEC. Deuterium exchange experiments in ²H₂O helped to assess the exposure of the amide group. Over time, NMR spectra showed decreased signal intensities in the amide (8–10.5 ppm) and aromatic (6–8 ppm) regions, reflecting protein aggregation. While SEC measured monomer loss, NMR slightly overestimated degradation rates based on aromatic signal intensities. Overall, NMR spectroscopy provides complementary insights for mAb formulation development and stability assessment.
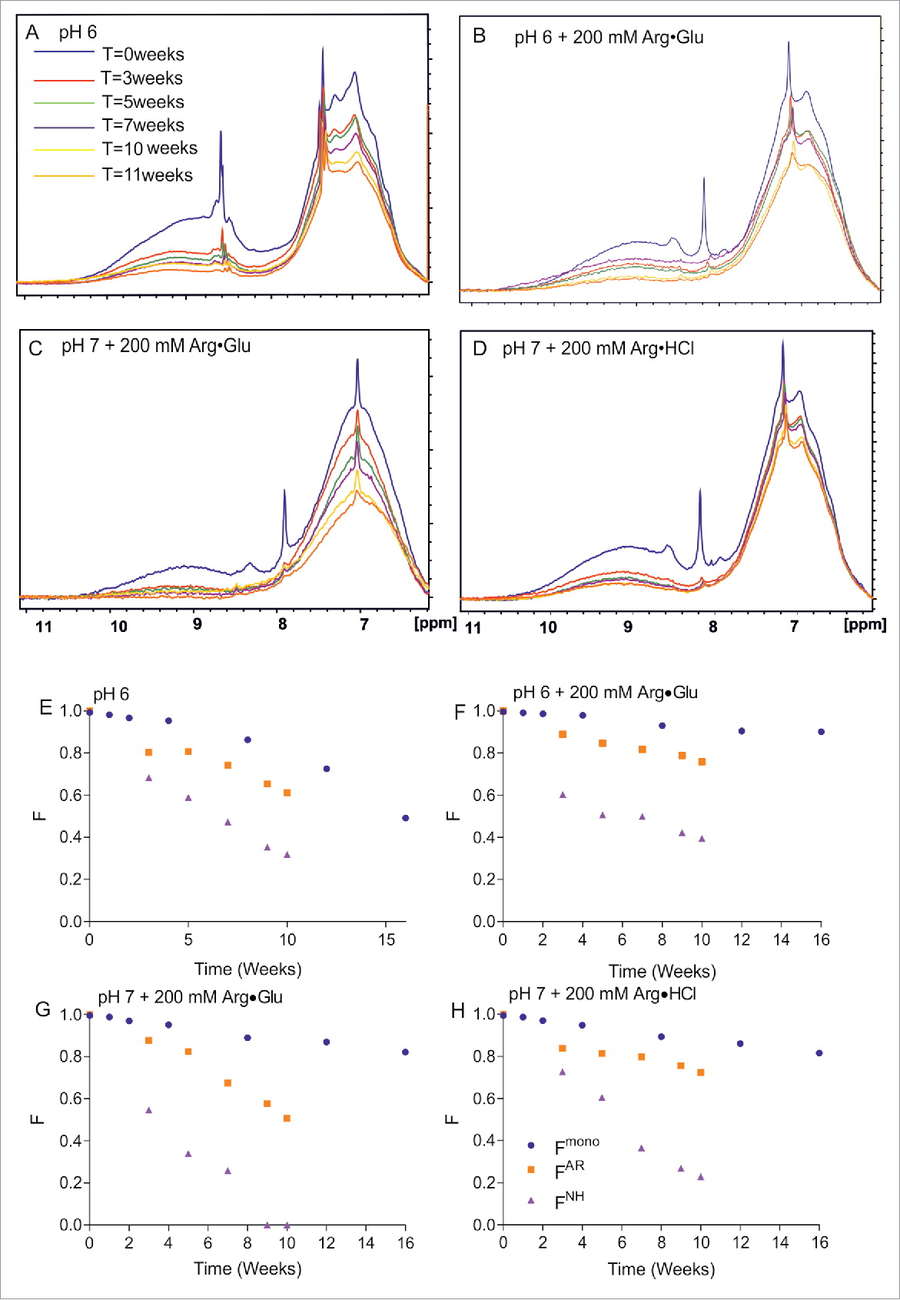 Figure 5. Assessing by NMR and SEC the long-term storage stability of mAb2 at 40°C in selected formulations. The 1D ¹H NMR spectral overlays (amide and aromatic region) for 4 different formulations of 300 mg/ml mAb2 in 10 mM citrate-phosphate buffer are shown, as a function of time: at pH 6 in the absence of additives (A); in the presence of 200 mM Arg·Glu (B); at pH 7 in the presence of 200 mM Arg·Glu (C); and in the presence of 200 mM Arg·HCl (D). Correspondent panels (E)-(H) show for the same 4 formulations the time-dependence of relative fractions of aromatic (FAR) and amide (FNH) signals remaining in the spectra vs time, reporting on soluble protein loss. Independently, the fraction of monomeric protein Fmono was assessed using SEC and plotted. (Kheddo et al., 2016)
Figure 5. Assessing by NMR and SEC the long-term storage stability of mAb2 at 40°C in selected formulations. The 1D ¹H NMR spectral overlays (amide and aromatic region) for 4 different formulations of 300 mg/ml mAb2 in 10 mM citrate-phosphate buffer are shown, as a function of time: at pH 6 in the absence of additives (A); in the presence of 200 mM Arg·Glu (B); at pH 7 in the presence of 200 mM Arg·Glu (C); and in the presence of 200 mM Arg·HCl (D). Correspondent panels (E)-(H) show for the same 4 formulations the time-dependence of relative fractions of aromatic (FAR) and amide (FNH) signals remaining in the spectra vs time, reporting on soluble protein loss. Independently, the fraction of monomeric protein Fmono was assessed using SEC and plotted. (Kheddo et al., 2016)
Antibodies as Therapeutic Agents
Antibodies have become a cornerstone of modern drug development. They are used in targeted therapies for diseases such as cancer, autoimmune disorders, and infections.
- Targeting Specific Pathogens or Cancer Cells: Therapeutic antibodies can be designed to specifically target pathogens, cancer cells, or abnormal proteins, providing highly targeted and effective treatments.
- Enhancing Immune Response Through Antibody-Mediated Therapies: Monoclonal antibodies can also enhance the immune response by triggering immune mechanisms such as antibody-dependent cellular cytotoxicity and complement activation.
Creative Biostructure offers expert services in X-ray Crystallography, Cryo-Electron Microscopy (Cryo-EM), NMR Spectroscopy, and Atomic Force Microscopy (AFM), supporting your antibody structure research and drug development efforts. Contact us today to discuss your project!
References
- Fesseha H, Degu T, Endashaw D. Therapeutic application of monoclonal antibodies: A review. JLSB. 2020;10(5):59-69. doi:10.51145/jlsb.2020.8
- Jay JW, Bray B, Qi Y, et al. IgG antibody 3D structures and dynamics. Antibodies. 2018;7(2):18. doi:10.3390/antib7020018
- Kheddo P, Cliff MJ, Uddin S, Van Der Walle CF, Golovanov AP. Characterizing monoclonal antibody formulations in arginine glutamate solutions using ¹H NMR spectroscopy. mAbs. 2016;8(7):1245-1258. doi:10.1080/19420862.2016.1214786
- Scapin G, Yang X, Prosise WW, et al. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat Struct Mol Biol. 2015;22(12):953-958. doi:10.1038/nsmb.3129
- Immunobiology (5th Edition, 2001). Garland Pub; Janeway C. ISBN-10: 0443070989
- Molecular biology of the cell (6th Edition, 2015). Garland Science; Taylor and Francis group. ISBN-10: 9780815344322