Structural Research of Light-Harvesting and Reaction Center Complexes
In the field of photosynthesis research, understanding the intricate details of light-harvesting and reaction center complexes is of paramount importance. These complexes play a crucial role in capturing and converting light energy into chemical energy, driving the fundamental process of photosynthesis in purple bacteria. Among the various purple phototrophic bacteria, Rhodobacter (Rba.) sphaeroides stands out as the most extensively studied and widely used model organism due to its metabolic diversity, ease of growth, and well-established genetics.
Structural Diversity of Light-Harvesting and Reaction Center Complexes
The core light-harvesting (LH1) system in Rba. sphaeroides exhibits a remarkable structural diversity, featuring both dimeric and monomeric forms that correspond to S- and C-shaped structures observed in native membranes, respectively. The LH1 complex, which surrounds the reaction center (RC), is composed of 14 αβ-polypeptides and possesses a large ring opening. Recent research has focused on characterizing the monomeric LH1-RC complex from Rba. sphaeroides strain IL106. Through advanced cryo-electron microscopy (cryo-EM) techniques, the team successfully determined the C-shaped structure of the monomeric LH1-RC complex, providing valuable data for a deeper understanding of its functional roles.
The Dimeric LH1-RC Complex: Symmetry and Asymmetry
Dimeric LH1-RC complexes have been of great interest to researchers in the field. Previous studies reported a two-fold symmetric structure for an LH2-deficient mutant strain of Rba. sphaeroides (strain DBCΩG). Moreover, similar dimeric complexes were observed not only in Rba. blasticus and Rhodobaca bogoriensis but also in a few other purple bacteria.
In a recent breakthrough, researchers utilized cryo-EM to explore the native dimeric core complex from Rba. sphaeroides IL106. Surprisingly, they discovered that the native LH1-RC dimer exhibits an asymmetric S-shaped structure, challenging the previously reported two-fold symmetric model. This finding underscores the importance of continuous research and the necessity for comprehensive investigations to grasp the complexities of photosynthetic systems accurately.
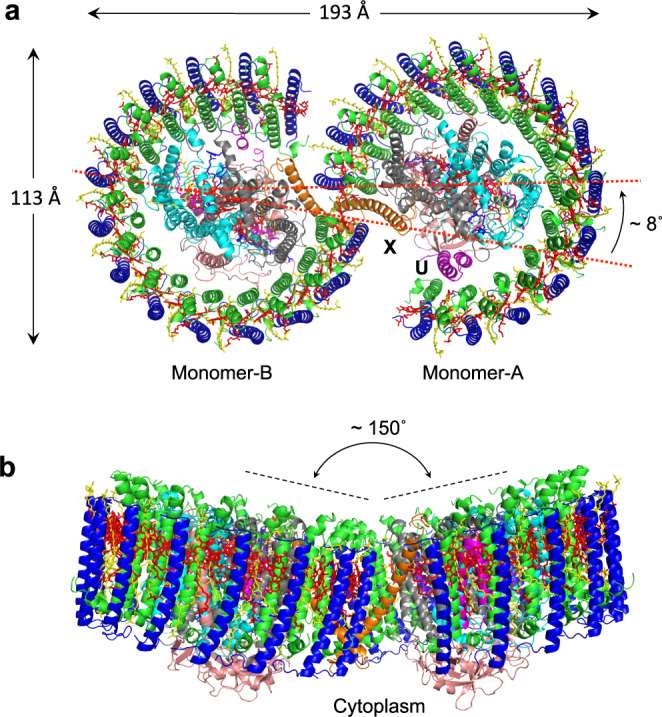 Figure 1. Structure overview of the dimeric LH1-RC complex from Rba. sphaeroides IL106. (Tani K, et al., 2022)
Figure 1. Structure overview of the dimeric LH1-RC complex from Rba. sphaeroides IL106. (Tani K, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| LH1-RC complex, P21 crystal form | Thermochromatium tepidum | X-ray diffraction | 3.01 Å | 4V8K |
| LH1-RC complex | Thermochromatium tepidum | X-ray diffraction | 1.90 Å | 5Y5S |
| LH1-RC complex with bound electron donor HiPIP | Thermochromatium tepidum | X-ray diffraction | 2.89 Å | 7C52 |
| Photosynthetic assembly RC-FMO2 | Chlorobaculum tepidum TLS | Cryo-EM single particle analysis | 3.08 Å | 7UEB |
| LH1-RC complex | Blastochloris viridis | Cryo-EM single particle analysis | 2.87 Å | 6ET5 |
| RC-LH core complex | Roseiflexus castenholzii | Cryo-EM single particle analysis | 4.10 Å | 5YQ7 |
| RC-LH114-W complex | Rhodopseudomonas palustris CGA009 | Cryo-EM single particle analysis | 2.65 Å | 6Z5S |
| LH1-RC super complex | Rhodospirillum rubrum, Rhodospirillum rubrum ATCC 11170 | Cryo-EM single particle analysis | 2.76 Å | 7EQD |
| LH1-RC super complex | Rhodospirillum rubrum ATCC 11170 | Cryo-EM single particle analysis | 2.50 Å | 7OY8 |
| LH1-RC super complex | Cereibacter sphaeroides | Cryo-EM single particle analysis | 2.94 Å | 7F0L |
| RC-LH1-PufXY monomer complex | Cereibacter sphaeroides 2.4.1 | Cryo-EM single particle analysis | 2.50 Å | 7PIL |
| LH1-RC super complex dimer | Cereibacter sphaeroides f. sp. denitrificans | Cryo-EM single particle analysis | 2.75 Å | 7VY2 |
| LH1-RC super complex dimer, in class 1 | Cereibacter sphaeroides 2.4.1 | Cryo-EM single particle analysis | 2.74 Å | 7VOR |
| Photosynthetic FMO-Reaction Center complex (FMO-RC) | Chlorobaculum tepidum TLS | Cryo-EM single particle analysis | 2.50 Å | 7Z6Q |
Table 1. Structural research of light-harvesting and reaction center complexes.
Creative Biostructure has established itself as a leader in providing high-quality structural analysis services for complex biomolecules. Leveraging cutting-edge techniques, such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryo-electron microscopy (cryo-EM), our expert team of scientists excels in unraveling the intricate structures of membrane protein complexes. Contact us to explore how our advanced capabilities can empower your research and propel you towards realizing your scientific aspirations.
References
- Tani K, et al. Asymmetric structure of the native Rhodobacter sphaeroides dimeric LH1–RC complex. Nature Communications. 2022, 13(1): 1904.
- Xie H, et al. Cryo-EM structure of the whole photosynthetic reaction center apparatus from the green sulfur bacterium Chlorobaculum tepidum. Proceedings of the National Academy of Sciences. 2023, 120(5): e2216734120.