Structural Research of Betanodavirus
Betanodavirus, also known as nervous necrosis virus (NNV), is a genus of enveloped positive-stranded RNA viruses in the family Nodaviridae. Betanodavirus infects more than 50 species of marine and freshwater fish, especially juveniles, and causes viral neuro-necrosis (VNN) and viral encephalopathy and retinopathy (VER). In recent years, scientists have endeavored to obtain high-resolution structural details of Betanodavirus to gain insights into the molecular mechanisms of viral assembly and infection and to provide a structural basis for vaccine development.
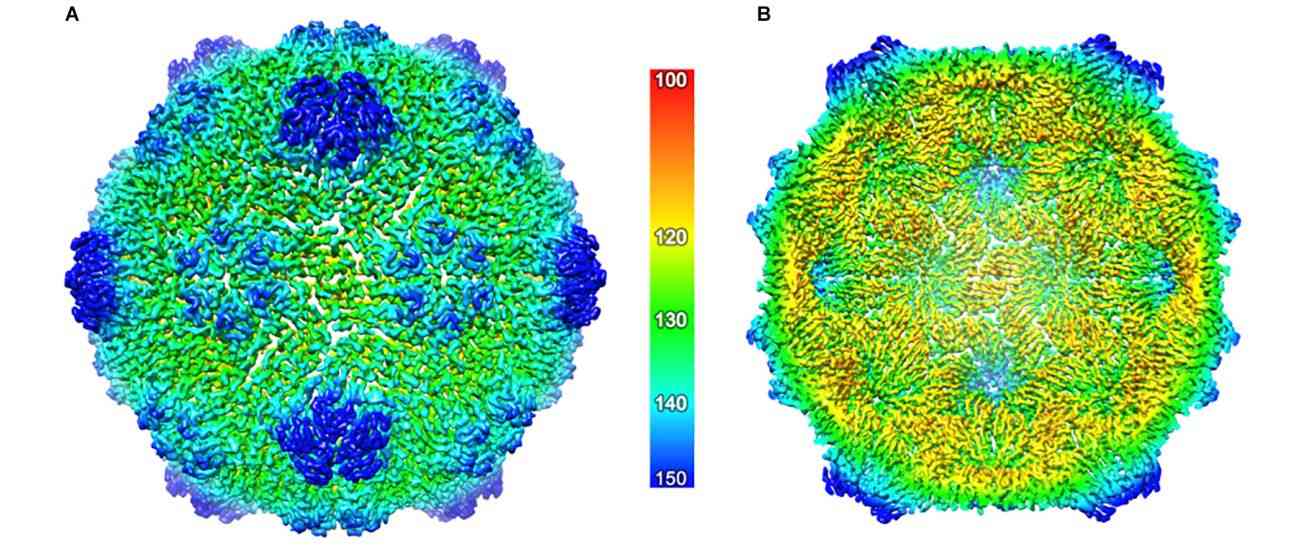 Figure 1. The cryo-EM structure of the ACNNV capsid. (Marsian J, et al., 2019)
Figure 1. The cryo-EM structure of the ACNNV capsid. (Marsian J, et al., 2019)
Structural Composition of Betanodavirus
Betanodavirus is a small, unenveloped virus, approximately 25-30 nm in diameter, with T = 3 icosahedral symmetry (180 copies of a single protein). The viral genome consists of two single-stranded positive-sense RNA molecules, called RNA1 and RNA2, which are co-packaged into a single viral particle. The 5' ends of these RNAs were capped, but their 3' ends were not polyadenylated. The largest fragment, RNA1, consists of approximately 3100 nucleotides (nt) and contains the open reading frame (ORF) for the RNA-dependent RNA polymerase (RdRp) (also known as protein A). RNA2, the smallest fragment, encodes capsid protein (CP). In addition, a subgenomic RNA, called RNA3, which is not packaged into viral particles, is synthesized from the 3' end of RNA1 and encodes two nonstructural viral proteins, B1 and B2.
Research Progress on the Structure of Betanodavirus
In recent years, researchers have characterized the crystal structures of various forms of the grouper neuron necrosis virus (GNNV) of the genus betanodavirus: complete T = 3 GNNV-like particles (GNNV-LP) with a resolution of 3.6 Å; a mutant subviral particle with a T = 1 delta-P structural domain (SVP),3.1 Å; N-ARM deletion mutants at 7.0 Å; and single P structural domains of GNNV CP at 1.2 Å. The crystal structure of GNNV-LP suggests some significant and unique variations in the molecular mechanisms of capsid structure and capsid assembly. The various forms of T = 3 and T = 1 GNNV capsids suggest that the N-terminal arginine-rich motif (N-ARM) acts as a molecular switch. In addition, the P structural domain and its DxD motif, together with two bound Ca2+ ions, play a critical role in the trimerization and particle assembly of GNNV CP.
Structural information on virus-like particles is essential to aid in vaccine design, modification, or remodeling. Cryo-electron microscopy (cryo-EM) allows visualization of macromolecules in a natural state and has been used to obtain structural information on many virus particles. Combined with three-dimensional (3D) reconstruction techniques, cryo-EM images of macromolecules can be used to research their high-resolution structures and interactions, which can help to reveal the mechanisms of viral capsid assembly and invasion.
Creative Biostructure, as a leader in structural analysis of biological macromolecules, offers a comprehensive range of virus-like particles (VLPs) products and solutions for viral structure analysis. Our VLPs are safe and non-infectious tools for vaccine research, providing researchers with an efficient platform for vaccine and antiviral drug discovery.
| Cat No. | Product Name | Virus Family | Source | Composition |
| CBS-V548 | Betanodavirus VLP (CP Proteins) | Nodaviridae | E. coli recombinant | CP (Capsid Protein) |
| Explore All Betanodavirus VLP Products | ||||
In addition, Creative Biostructure offers comprehensive viral structure resolution services, including sample preparation, data collection and analysis, and structure determination to satisfy clients' specific requirements. Our cutting-edge cryo-electron microscopy (cryo-EM), coupled with extensive experience in viral structure research, ensure that high-quality results are delivered in a timely and cost-effective manner.
Please feel free to contact us to learn more about our viral structure analysis services. Our team of expert scientists is always glad to answer your questions and assist you in achieving your research goals.
References
- Marsian J, et al. Plant-Made Nervous Necrosis Virus-Like Particles Protect Fish Against Disease. Front Plant Sci. 2019. 10: 880.
- Bandín I, Souto S. Betanodavirus and VER Disease: A 30-year Research Review. Pathogens. 2020. 9(2): 106.
- Xie J, et al. Structural analysis and insertion study reveal the ideal sites for surface displaying foreign peptides on a betanodavirus-like particle. Vet Res. 2016. 47: 16.
- Chen NC, et al. Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection. PLoS Pathog. 2015. 11(10): e1005203.
