Custom MemPro™ Single-helix ATPase Regulators
Creative Biostructure has developed custom MemPro™ gene-to-structure services for single-helix ATPase regulators. Single-helix ATPase regulators belong to the bitopic protein superfamilies with alpha-helical transmembrane anchors and regulate ATPases. Generally alpha-helices and beta-barrels are the main components of the transmembrane domains in transmembrane proteins. Single-helix ATPase regulators include a total of 10 proteins from 2 families: calcium ATPase regulators and FXYD regulators.
Calcium ATPase regulators locate in endoplasm reticulum (ER) and regulate calcium homeostasis. The members include phospholamban (monomer/pentamer, Homo Sapiens, Sus Scrofa, Oryctolagus Cuniculus), sarcolipin (Homo Sapiens) and cytoplasmic helix (Homo Sapiens).
FXYD regulators locate in the plasma of eukaryo and have the Na, K-ATPase function regulation property (phospholemman) (Homo Sapiens). Common examples include Na, K-ATPase regulatory protein FXYD4 (Rattus Norvegicus) and Na, K-ATPase regulatory protein FXYD2 (Homo Sapiens).
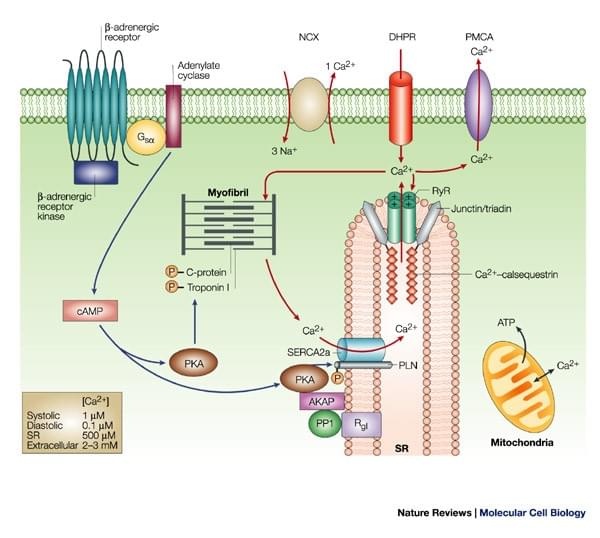 Figure. Phospholamban regulates calcium homeostasis and cardiac contractility
Figure. Phospholamban regulates calcium homeostasis and cardiac contractility
There are two kinds of calcium ATPase that transfers calcium, plasma membrane Ca2+ ATPase (PMCA) and sarcoplasmic reticulum Ca2+ ATPase (SERCA). Phospholamban (PLN, PLB) is an integral sarcoplasmic reticulum (SR) membrane protein containing 52 amino acids. It is mainly located in cardiac muscle, slow-twitch skeletal, endothelial cells, smooth muscles and in the hart, it has higher expression level in ventricles. PLB can be phosphorylated at three sites: serine-16 by CAMP-dependent protein kinase; serine-10 by Ca2+ phospholipid-dependent protein kinase and threonine-17 by Ca2+ calmodulin-dependent protein kinase A. Functionally, it reversibly inhibits the Ca2+ affinity and cardiac contractility of SERCA. Sarcolipin (SLN) is another small molecular protein regulating the sarco/endoplasmic reticulum Ca2+-ATPase (Serca) pump. SLN has similar protein sequences and structure to PLB. They both have three domains, luminal domain, transmembrane domain and cytoplasmic domain. While compared to PLB, SLN is expressed at higher levels in the atria instead of ventricles.
FXYD proteins are a new class of Na-K-ATPase regulators. In mammals, the FXYD protein family has seven members, FXYD1 (phospholemman, type I membrane protein), FXYD2 (the γ-subunit of Na-K-ATPas, type I membrane protein, two variants FXYD2a and FXYD2b), FXYD3 (mammary tumor marker Mat-8, two transmembrane domains), FXYD4 (cortico-steroid hormone-induced factor CHIF, type I membrane protein), FXYD5 (related to ion channel RIC or dysadherin), FXYD6 (phosphorhippolin) and FXYD7 (type I membrane protein). All FXYD family proteins have transmembrane domain and a FXYD motif. There are two conserved glycine residues located in the transmembrane domain.
Creative Biostructure can provide custom MemPro™ gene-to-structure services for membrane proteins. Please click for more information.
MacLennan DH and Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003 Jul; 4 (7):566-77.
Ka¨thi Geering. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol. 2006 Feb; 290(2):F241-50.
